Supplementary Information (doc 1082K)
advertisement

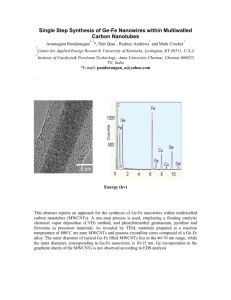
Supporting Information 3D Networked Carbon Nanotube/Diamond Core-Shell Nanowires for Enhanced Electrochemical Performances Seung-Koo Lee1†, Min-Jung Song2†, Jong-Hoon Kim1, Tae-Seok Kan1, Young-Kyun Lim1, Jae-Pyoung Ahn3 and Dae-Soon Lim1★ 1: Department of Materials Science and Engineering, Korea University, Anam-dong 5-ga, Seongbuk-gu, Seoul 136-713, Republic of Korea 2: Department of Materials Science and Engineering, Yonsei University, 50, Yonsei-ro, Seodaemun-gu, Seoul 120-749, Republic of Korea 3: Advanced Analysis Center, Korea Institute of Science and Technology, P.O. Box 131, Cheongryang, Seoul 130-650, Republic of Korea †These ★ authors contributed equally to this work. Corresponding author: dslim@korea.ac.kr Phone: +82-2-3290-3272 Fax: +82-2-929-5344 Role of nanodiamond Figure S1 shows the role of nanodiamond on MWCNTs to produce the 3D networked BDD-CNT nanostructure. MWCNTs treated by the ESAND technique were fully coated with the BDD shell after diamond deposition for 60 min (Figure S1 (c)). However, the MWCNTs left untreated by ESAND were transformed to sp2 graphite clusters after diamond deposition (Figure S1 (b)). In the hydrogen-rich environment, atomic H etched the graphitic sp2 carbon phase. When the activated atomic H meets sp2 carbon atoms, they react with each other and are removed in the form of a gaseous hydrocarbon or produce graphite clusters on the surface. Thus, densely attached nanodiamonds on the MWCNT surface were required to fabricate the BDD-CNT core-shell nanostructures. Figure S1. SEM images of (a) the as-grown MWCNTs, (b) the sp2 carbon clusters transformed from MWCNTs left untreated by ESAND, and (c) the 3D randomly networked BDD-CNT nanostructures obtained from MWCNTs treated by ESAND. (Scale bars: (a) 1 m, (b, c) 10 m) S2 Dispersion of nanodiamond Commercial nanodiamond particles were used to synthesize the BDD-CNT core-shell structures. The as-purchased nanodiamond particles with the primary particle size of 5–10 nm agglomerated spontaneously into a size of about 1 m to reduce their surface energy. Thus, to attach the nanodiamond particles on the thin MWCNTs, a dispersion process was required. Figure S2 shows the dispersion of the nanodiamond particles. Before the attrition milling process, 0.5 g of the nanodiamonds and 50 mL of 35 wt.% PSS solution were mixed in 300 mL of DI water. The vessel was filled to 70% with 0.1 mm sized zirconium oxide beads. The ceramic impellers were rotated at 1000 rpm for 6 h. After the attrition milling process, the particle size and zeta potential were measured by a zeta potential analyzer (Otsuka Electronics, ELSZ-1). Figure S2. Dispersion of the agglomerated nanodiamond particles. After the milling process, the average particle size and zeta potential were 13.8 nm and -55.7 mV, respectively. Since the nanodiamond particles consisted of a positive surface charge, the anionic PSS spontaneously covered the nanodiamond surface. In addition, the harsh mechanical milling process reduced the size of the agglomerated nanodiamond particles from ~1 m to ~10 nm. This allowed for the attachment of the nanodiamond particles onto the MWCNT surfaces. The high negative zeta potential of -55.7 mV induced strong electrostatic bonding between the cationic PDDA-covered MWCNTs and PSS-NDs. S3 Raman study of the BDD-CNT core-shell structure Raman spectroscopy was carried out on the MWCNTs, the BDD film, and the BDD-CNT core-shell structures (Figure S3). The MWCNTs exhibited a clear graphitic D-band around 1350 cm-1 and a G-band around 1603 cm-1. In addition, the BDD film, synthesized under the identical deposition conditions as that used for the BDD-CNT, showed typical peaks of a sp3 diamond phase at 1332 cm-1 and nanocrystalline diamond (NCD) peaks at 1150 cm-1 and 1480 cm-1.S1. However, the BDDCNT core-shell structure synthesized showed all the peaks corresponding to the MWCNTs (i.e., the D-band and G-band) and that corresponding to BDD related to sp3 diamond and nanocrystalline diamond (NCD) phases. Figure S3. Raman spectra of the CNT, BDD, and BDD-CNT electrodes. S4 Immobilization of glucose oxidase on the electrodes The procedure used for the immobilization of glucose oxidase on the BDD-CNT electrode is shown in Figure S4. Conventional silanization methodsS2 were used to immobilize enzymes covalently to BDD-CNT electrodes. To characterize the electrochemical performance of the BDD-CNT core-shell electrode, BDD and CNT electrodes were used as control electrodes in this study. The oxidation of CNT, BDD, and BDD-CNT hybrid electrodes was achieved with an oxygenplasma system (TePla 100-E). The oxidation parameters were O2 pressure = 0.14 kPa, plasma power = 200 W and time = 5 min. The static water contact angle after the oxidation was below 5°. All the electrodes were successfully incubated for 90 min. in 3% solution of 3-aminopropyltriethoxysilane (3-APTES) in ethanol/water (v/v, 95/5) and thoroughly rinsed with ethanol and DI water. After rinsing, the electrodes were dried with N2 gas. The silanized electrodes were further incubated in a vacuum oven for 30 minutes at 120 °C to enhance the stability of the silane layer. The electrodes were further modified with gluta-aldehyde (GA) for the binding of APTES to the enzyme by exposing the electrode surface to 25% GA solution for 1 h. Then, the GA-activated electrodes were rinsed with water and dried by N2 gas. In the final step, the amide bonds between the N-terminal of the enzyme molecule and the GA-activated silanized electrodes were formed by dipping the electrode in a 50 mM phosphate buffer (PB) solution (pH 7.0) containing the enzyme for 24 h. After the enzyme layer was formed, the electrodes were rinsed by streaming PB solution to remove residual monomers or weakly linked enzyme molecules. Figure S4. Schematic illustrating the immobilization of glucose oxidase on BDD-CNT electrodes. S5 Reproducibility of the glucose sensor based on the BDD-CNT electrode Table S1. The reproducibility of the glucose sensor based on the BDD-CNT electrode. Current [μA] Concentration [μM] R.S.D. [%] Sensor #1 Sensor #2 Sensor #3 Mean 0.000654 2.780 2.625 2.579 2.661 3.962 0.00262 2.813 2.779 2.626 2.739 3.640 0.0105 2.903 2.944 2.772 2.873 3.127 0.0419 3.225 3.212 3.116 3.184 1.862 0.0838 3.502 3.342 3.429 3.424 2.339 S6 References [S1] Pfeiffer, R., Kuzmany, H., Knoll, P., Bokova, S., Salk, N. & Günther, B. Evidence for trans-polyacetylene in nanocrystalline diamond films. Diam. Relat. Mater. 2006, 12, 268-271. [S2] Sarath Babu, V. R., Kumar, M. A., Karanth, N. G. & Thakur, M. S. Stabilization of immobilized glucose oxidase against thermal inactivation by silanization for biosensor applications. Biosens. Bioelectron. 2004, 19, 1337-1341. S7