Sensors & Transducers Magazine, Vol.40, Issue 2, 2004, pp.152-161
Sensors & Transducers
ISSN 1726- 5479
© 2004 by IFSA
http://www.sensorsportal.com
In Situ Monitor the Adsorption of Triton X-100 on Quartz Surface by an
Electrode-Separated Piezoelectric Sensor
Dazhong Shen, Fei Wang, Qi Kang, Duanling Yang
School of Chemistry and Chemical Engineering, Shandong University, Jinan, 250100, P.R. China,
Fax: +86-0531-8564464, e-mail: dzshen@sdu.edu.cn
Received: 17 December 2003
/Accepted: 14 February 2004
/Published: 20 February 2004
Abstract: In the configuration of an electrode-separated piezoelectric sensor (ESPS), the quartz crystal
surface is in a direct contact with the liquid phase. The quartz surface can be hydrated to form silanol
groups, which can adsorb nonionic surfactant Triton X-100 through hydrogen-bonding interaction
between the silanol OH group and the ether oxygen atoms. The adsorption process of Triton X-100 on
quartz crystal surface corresponds to a mass increase and can be monitored in situ by the ESPS device.
And the adsorption densities and kinetics parameters can be obtained in the ESPS method. It was
shown that the adsorption of Triton X-100 on quartz surface is reversible with respect to the dilution of
bulk phase and can be analyzed using Langmuir model. The adsorption density and adsorption
equilibrium constant decrease as pH rises. With increasing ionic strength, the adsorption rate constant
and equilibrium constant increase slightly.
Keywords: piezoelectric sensor, quartz crystal surface, ESPS device
1. Introduction
The nature of the adsorption of surfactants, and especially nonionic surfactants, on solid surfaces is of
considerable interest because of their importance to a number of industrial and technological processes
associated with colloidal stability and detergency1-2. The adsorption is the result of one or more
152
Sensors & Transducers Magazine, Vol.40, Issue 2, 2004, pp.152-161
contributing forces arising from electrostatic attraction, chemisorption, hydrogen bonding,
hydrophobic chain-chain interaction, and solvation. Understanding the adsorption mechanism of
surfactant molecules at the solid-liquid interface is an important way toward modeling industrial
processes which use surfactants on a large scale, such as detergency, water treatment, oil recovery, ore
refinement by flotation, painting cosmetics, agrochemical formulations and textiles. In addition,
adsorption behavior can give useful clues to the chemistry of the surfactant molecules themselves.
In most of the studies related to surfactant adsorption, a solution depletion method was used 3-5. This
method provides mainly the information of equilibrium adsorption densities. However, the information
of adsorption kinetics and adsorption layer is also important for understanding the adsorption process.
The adsorption of substance from aqueous solutions on various surfaces has been investigated using
different techniques, such as neutron reflection6, ellipsometry7, atomic force microscopy8, surface
plasmon resonance9, small-angle neutron scattering10, slab optical waveguide11, infrared12, and quartz
crystal microbalance (QCM)13.
The QCM has emerged as a powerful technique for in situ monitor the mass changes at solid/liquid
interface. Under certain experimental conditions, the oscillating frequency of the QCM decreases
linearly with increasing adsorption density. The adsorption density and kinetics parameters can be
estimated from the frequency shifts in the adsorption process. The QCM comprises a thin, vibrating,
AT-cut piezoelectric quartz crystal disc sandwiched between two metal-film electrodes that provide an
alternating electric field, which induces a shear vibration of the quartz crystal disc with resonant
frequencies in the MHz region. Hence, the QCM is used mainly to measure the mass change on the
surface of the metal film electrode 14.
In the construction of an electrode-separated piezoelectric sensor (ESPS)15, a quartz crystal surface is
in a direct contact with the liquid phase. The ESPS has been applied to monitor the adsorption
processes of ionic surfactants16-17, metallic ion18-19, proteins20-21, anionic surfactant22 onto quartz
surface. In this paper, the adsorption process of nonionic surfactant on quartz surface was monitored
with the ESPS as a sensitive surface mass technique. Triton X-100 is chosen as model nonionic
surfactants as it is one of the most popular nonionic surfactants and was widely used in
immunochemistry. During the adsorption process of Triton X-100 on quartz crystal disc surface, the
frequency of the ESPS decreases with increasing adsorption mass. The technique is able to provide
adsorption density and kinetics information. The influence of pH and ionic strength on the adsorption
parameters is also investigated.
2. Experimental
2.1 Apparatus and materials
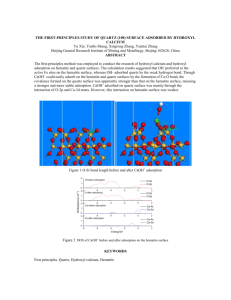
The configuration of the ESPS used was illustrated in Fig.1. A 5 MHz AT-cut quartz crystal disc of
25.4 mm in diameter was used. A gold electrode with a piezoelectric active area of 1.1 cm2 was
evaporated on one side of the quartz disc. The quartz disc was fixed to a glass detection cell by silicon
glue with the side of bare quartz facing the liquid phase and another side with the gold electrode in the
air. A platinum disk electrode with a diameter of 8 mm was used as the separated-electrode. The
153
Sensors & Transducers Magazine, Vol.40, Issue 2, 2004, pp.152-161
distance between the separated-electrode and the quartz crystal disc was 2 mm. The
separated-electrode and gold electrode were connected to an oscillator circuit. The oscillating
frequencies of the ESPS were recorded by a universal frequency counter (7201,Shijiazhuang No.4
Radio Factory) connected to a PC computer. A spectrometer (Lambda 35, Perkin-Elmer Instruments)
was used to determine the concentration of Triton X-100. The experiments were preformed in room
temperature at 251C. Each experiment was carried out three times and the averaged value was
reported.
1
2
5
3
6
4
7
Fig.1 Experimental setup for monitor adsorption process on quartz crystal surface by the ESPS method.
(1) Oscillator circuit, (2) frequency counter; (3) computer; (4) quartz crystal and gold electrode;
(5) platinun separated electrode (6) stirrer ; (7) Triton X-100 solution
Analytical-reagent grade chemicals and Mini-Q water were used. Triton X-100 was purchased from
Sigma Chemical Co. and was used without further purification. Quartz powder was purchased from
No.2 Shanghai Regent Manufacture. The specific surface area of quartz powder was determined by the
BET adsorption method to be 1.2 m2.g-1. Triton X-100 stock solutions were prepared by the measuring
medium used in adsorption experiments. The conductivity of the stock solution and the measuring
medium were adjusted the same for each adsorption experiment.
2.2. Adsorption on quartz crystal surface in ESPS method
Before the determination of adsorption, the quartz crystal disc surface was cleaned with 0.1 mol/L
NaOH, following by 0.1 mol/LHCl, then rinsed thoroughly with water then measuring medium. 50 ml
of measuring medium was added in the detection cell. After 30 min of stabilization, the oscillating
frequency of the ESPS was measured with an integral time of 10 s under stirring. The stable oscillating
frequency of the ESPS, F1, was recorded as the reference. A certain amount of Triton X-100 stock
solution was added into cell, the oscillating frequencies, F2, were recorded as a function of adsorption
time. The shifts in the oscillating frequencies, F= F2F1, were used for the estimation of adsorption
rate constants and adsorption density.
154
Sensors & Transducers Magazine, Vol.40, Issue 2, 2004, pp.152-161
2.3 Adsorption on quartz powder surface in solution method
2 g quartz powder was mixed with a 30 ml Triton X-100 solution prepared by the 0.1 mol/L NaCl
+0.01 mol/L HCl until equilibration of the system was reached. After centrifugation, the concentration
of Triton X-100 in the clear supernatant was determined by a spectrophtometric method at the
wavelengths of 277 nm. Adsorption densities were estimated from the decrease in the concentration
after agitation with quartz powder.
3. Results and Discussion
3.1 Adsorption of Triton X-100 on quartz surface
Adsorption of surfactants at solid/liquid interface is the result of one or more contributing forces
arising from electrostatic attraction, chemisorption, hydrogen bonding, hydrophobic chain-chain
interaction, and solvation effects23. For the nonionic surfactant adsorption, hydrogen bonding
mechanisms may be considered as the initial driving force the adsorption24.
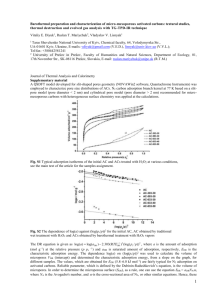
As shown in Fig.2, the oscillating frequency of the ESPS decreases after the addition of Triton X-100.
The decrease in the oscillating frequency of the ESPS indicates the increases in the adsorption density.
Because of the absence of ionic and chemisorption, hydrogen bonding mechanisms may be considered
as the main driving force for the adsorption of Triton X-100 on quartz interface. The adsorption of
Triton X-100 on quartz crystal surface of the ESPS results in an increase in the mass load of the
oscillating quartz crystal. The oscillating frequency of the ESPS decreases as the mass load increase.
Hence, the adsorption densities can be in situ monitored. From the time responses of the ESPS, the
adsorption rate constants can be estimated.
Triton X-100
0
F (Hz)
-15
-30
1
2
-45
3
4
-60
0
60
120
180
240
300
Time (s)
Fig.2 Frequency shifts of the ESPS with adsorption time during the adsorption process of Triton
X-100 on quartz surface. Measuring medium is 0.01 mol/LHCl + 0.1 mol/LNaCl. Concentration of
Triton X-100 : (1) 0.50×10-5 M; (2) 1.0×10-4 M; (3) 2.0 ×10-4 M; (4) 5.0×10-4M.
The lines are the regression results using Eq.(5)
155
Sensors & Transducers Magazine, Vol.40, Issue 2, 2004, pp.152-161
It can be seen that the adsorption rate of Triton X-100 on quartz crystal surface is quite rapid. Under
our experimental conditions, the adsorption process approaches the equilibrium after 3 min in 5104
mol/LTriton X-100 solution. And the adsorption rate decreases at lower concentration of Triton X-100
solution. After the adsorption, quartz crystal was rinsed thoroughly with surfactant-free measurement
medium, the oscillating frequency can be returned back its previous baseline (data were not shown in
the Figure). It was indicated that the adsorption of Triton X-100 onto quartz surface is reversible with
respect the dilution of the bulk phase.
It was shown that the frequency of the ESPS is also related to the conductivity, permittivity, density
and viscosity bulk liquid phase 25. Hence, these liquid parameters must be kept constant in an
adsorption experiment. As the concentration of Triton X-100 is low, these parameters is not influence
by the addition of Triton X-100, which were verified by the measurements of the four properties of
Triton X-100 in the concentration used in this paper. Hence, The frequency shift of the ESPS can be
attributed to the change in adsorption mass of Triton X-100 on quartz surface.
3.2 Dependence of adsorption density on bulk concentration
According to the Sauerbrey equation26, the adsorption density shows a linear correlation with the
frequency decrease in the adsorption process:
= m/A= -F/2.26×10-6F02,
(1)
where is the adsorption density in g/cm2 , m is the mass adsorbed onto quartz crystal. F and F0 are
the frequency shift and the fundamental frequency of the quartz crystal in Hz, respectively. For
F0=5106Hz used in the paper, the frequency decrease of 1Hz corresponds to the mass increase of 17.8
ng/cm2 according to greater than the true adsorption density because the influence of surface roughness
of quartz surface.
As discussed previously20, the observed adsorption density estimated from Eqn.(1) was greater than
the true adsorption density because the influence of surface roughness of quartz surface. As can be
seen in Fig.3, the adsorption densities obtained in the ESPS method were significantly grater than
those determined using quartz powder in a solution depletion method. In addition, the shapes of the
two adsorption isotherm are similar and can be fitted using the Langmuir model in Eqn.(2).
=CK/(1+CK) ,
(2)
where is the saturation adsorption density, C is the equilibrium bulk concentration and K is the
adsorption equilibrium constant.
According to data in Fig.3, =0.961 ± 0.059 g/cm2 and K = (3.98±0.27)104 mol-1.L were obtained
in the ESPS method. But =0.197±0.016g/cm2 and K =(3.26±0.24) 104 mol-1.L were given in the
solution depletion method. In order to correct the influence of surface roughness on the adsorption
density in the ESPS method, a calibration coefficient was employed in Eqn.(1).
=m/A=-F/2.26×10-6F02,
(3)
156
Sensors & Transducers Magazine, Vol.40, Issue 2, 2004, pp.152-161
where is the ratio of in solution depletion method to the ESPS method . Under our experimental
conditions, =0.205(0.197/0.961) is used in Eq.(3) for estimation the adsorption densities in the ESPS
method. When Eq.(3) was used, the adsorption isotherms obtained by the ESPS and solution depletion
methods were very close. The value of is agreement with the result of 0.187g/cm2 in reference 27.
- 60
1.06
- 45
0.795
-30
0.530
2
-15
0
0
2
4
6
8
(g/cm2)
F (Hz)
1
0.265
0.00
10
Ceq (10-4 mol/L)
Fig.3 Comparision of the adsorption isotherm for Triton X-100 on quartz crystal
surface : (1) ESPS method in quartz crystal disc (2) solution depletion method in quartz
powder. The medium is 0.1 mol/L NaCl + 0.01 mol/L HCl. The lines of 1 and 2 are the
regression results using Eq.(2)
3.3 Influence of pH on Adsorption of Triton X-100 on Quartz
In the adsorption mechanism of nonionic surfactant on silica surface, hydrogen bonds are most
probable between the polar chain of the nonionic surfactant and the silanol groups of the silica28.
Hence, pH is likely to be an influence factor for the adsorption Triton X-100 on quartz. As can be seen
in Fig. 4, which shows the shift of the adsorption of Triton X-100 onto quartz with pH. As Fig.4
illustrates, the adsorption densities of Triton X-100 decreases with rise of pH in medium. In the
medium of pH >11, the adsorption of Triton X-100 is difficult to be detected by the ESPS method.
The pH dependence can be explained as follows.
As pointed out above, the adsorption mechanism of Triton X-100 adsorbs on the quartz surface is
mainly through hydrogen-bonding interaction between the oxygen atoms in ethyleneoxy hydrophilic
and surface silanol groups. The ether oxygen atoms are unlikely effected by the pH of solution. But
silanol groups in quartz surface, includes Si(OH), =Si(OH)2 and Si(OH)3, depend on pH of the
solution. As the pH rises, parts of the silanol groups are isolated. The isolated hydroxyl groups
involved in hydrogen-bonding interaction with water molecules. On the other hand, the isolated
hydroxyl groups are incapable of providing a proton. As a result, their ability of forming hydrogen
bonds with ether oxygen atoms disappear. Consequently, the ionization of silanol groups markedly
diminished the capacity of quartz to adsorb polyethylene oxide29. Hence, the adsorption densities and
equilibrium constant of Triton X-100 onto quartz decreased at higher pH medium. The maximum
157
Sensors & Transducers Magazine, Vol.40, Issue 2, 2004, pp.152-161
adsorption of Triton X-100 occurs at pH=2, which is close to the point of zero charge (pzc) of quartz.
The low adsorption of Triton X-100 in basic solution is important to decrease to its loss by rock
adsorption in oil recovery applications.
-60
0.216
1
F (Hz)
0.162
3
-30
0.108
-15
(g/cm2)
2
-45
0.054
4
0
0.0
2.5
5.0
7.5
0.00
10.0
-4
Ceq (10 mol/L)
Fig.4 Influence of pH on the adsorption isotherm of Triton X-100 on quartz crystal
surface. Measuring medium: (1) 0.1 mol/LNaCl + HCl, pH=2; (2) 0.1
mol/LNaAc+ 0.1 mol/LHAc, pH=4.7; (3) 0.1 mol/LNaCl + 0.01mol/Lphosphate
buffer, pH=7, (4) 0.1 mol/LNaCl + 0.01 mol/Lphosphate buffer, pH=9. The lines
are the regression results using Langmuir model in Eq.(5)
3.4 Influence of ionic strength on adsorption of Triton X-100 on quartz
Influence of ionic strength on adsorption of Triton X-100 on quartz is illustrated in Fig.5. In the
logarithmic abscissa, the adsorptions are in S type. It has been seen that the effects of ionic strength on
the adsorption plateau is not very obvious. But an increase of the ionic strength of the bulk shifts the
adsorption curves toward lower concentrations. The phenomena may be attributed to the decrease of
the critical micelle concentration of nonionic surfactants with increasing ionic strength1. The addition
of the electrolyte causes the dehydration of the oxyethylene chains and decreases the solubility of
nonionic surfactants.
3.5 Estimation Adsorption and Desorption Rate Constants
Based on the experimental results that the adsorption isotherm of Triton X-100 on quartz surface is in
the shape of Langmuir type and the adsorption is reversible with respect to the dilution of the bulk
phase. The Langmuir kinetics model is used to estimate the adsorption rate parameters. As derived
previously18, the frequency shift response of the ESPS during the Langmuir adsorption processes is
expressed as
158
Sensors & Transducers Magazine, Vol.40, Issue 2, 2004, pp.152-161
F
Fsat CK
1 exp[ (k a C k d )t ] ,
1 CK
(4)
-50
0.180
-40
0.144
(g/cm2)
F(Hz)
where ka and kd are the rate constants for adsorption and desorption, respectively. K is adsorption
equilibrium constant and t is the adsorption time. Fsat is the frequency shift that corresponding to the
adsorption density of the saturation adsorption layer. C is the concentration of adsorbed molecule.
3
-30
0.108
2
-20
0.072
1
-10
0.036
0
0.0153
0.00
0.0625
0.25
4.0
1.0
16
-4
Ceq (10 mol/L)
Fig.5 Influence of ionic strength on adsorption isotherm of Triton X-100 on quartz crystal
surface: Measuring medium: (1) 1 mol/LNaCl ; (2) 0.1 mol/LNaCl ; (3) water.
The lines are the regression results using Langmuir model in Eq.(5).
As shown in Fig.2, the response curves of the ESPS in the adsorption process can be fitted by the
model in Eqn.(4). According to this fitting model, the values of the observed adsorption rate constant
kobs=Cka+kd was obtained. The dependence of kobs on C is depicted in Fig.6. The good linearity between
kobs on C supports the use of Langmuir kinetics model in the concentrated range reported.
According to the model of kobs=kaC+kd, the values of ka and kd corresponds to the slope and intercept of
regressed liner of kobs vs C. The results are listed in Table 1. It can be seen that the desorption rate
constants vary slightly in different medium. The adsorption rate constant and adsorption equilibrium
constant increase with increasing ionic strength of the medium.
Table 1 Adsorption kinetics parameters for Triton X-100 on quartz surface
Measuring medium
0.1 mol/L NaCl, pH=2
1.0 mol/L NaCl
0.1 mol/L NaCl
Pure water
ka(mol1.L.s1)
98.5 7.4
135 8.9
87.7 7.1
56.1 7.3
kb (103 s1)
2.43 0.03
3.07 0.03
2.69 0.03
2.76 0.03
ka/kb(104 mol1.L)
4.05 0.03
4.40 0.03
3.26 0.03
2.03 0.03
159
Sensors & Transducers Magazine, Vol.40, Issue 2, 2004, pp.152-161
.15
1
-1
kobs (s )
.12
2
.09
3
.06
4
.03
0.00
0
2
4
6
8
10
C (10-4 mol/L)
Fig.6 The relationship between kobs and concentration of Triton X-100. Measuring medium:
(1) 1 mol/LNaCl; (2) 0.1 mol/LNaCl + 0.01 mol/LHCl; (3) 0.1 mol/LNaCl; (4) water. The
slops and intercepts of the lines correspond to the rate constants
of adsorption and desorption, respectively
4. Conclusions
The adsorption process of Triton X-100 on quartz crystal surface was monitored in situ from the
frequency shift of the ESPS device. The ESPS method offers the advantages of rapid, simple, and
convenient. After calibration, both the adsorption density and adsorption rate constant can be obtained
in the ESPS method. It was shown that the adsorption isotherms are in the Langmuir type in the
concentration range used. The adsorption processes is reversible with respect the dilution of the bulk
phase and can be fitted by using Langmuir kinetics model. The adsorption density decreases as the pH
rises. Both the adsorption rate constant and adsorption equilibrium constant increase with increasing
ionic strength.
Acknowledgement
This work is supported by the National Science Foundation of China (No.20275021) and Science &
Technology Project of Education committee of Shandong Province (NO. J01A03).
160
Sensors & Transducers Magazine, Vol.40, Issue 2, 2004, pp.152-161
Reference
[1] J. S. Martin, Nonionic Surfactants Physical Chemistry, 1987, New York.
[2] M. A. Proskurnin, V. B. Ivleva, N. Y. Ragozina, and E. K. Ivanova, Analytical Sciences, 2000, 16, 397
[3] P. L. Desbene, F. Poret, and C. Treiner, J.Colloid Interface Sci., 1997, 190, 350.
[4] W. K. Backhaus, E. Klumpp, H.D. Narres, and M.J. Schwuger, J.Colloid Interface Sci., 2001, 242, 6.
[5] Y.H. Shen, Chemosphere, 2001, 44, 989.
[6] J. Penfold, E.J. Staples, I. Tucker, and R.K. Thomas, Langmuir 2000, 16, 8879
[7] F. Tiberg, and T. Ederth, J. Phys. Chem. B 2000, 104 , 9689.
[8] S. Manne, J.P. Cleveland, H.E. Gaub, G.D. Stucky, and P.K. Hansma, Langmuir 1994, 10, 4409.
[9] F.Caruso, T.Serizawa, D.N. Furlong and Y. Okahata, Langmuir 1995,11,1546
[10]P.G. Cummins, E.Staples and J. Penfold, J. Phys. Chem., 1991, 95, 5902.
[11]J.H. Santos, N.Matsuda, Z. Qi, T.Yoshida, A.Takatsu, and K.Kato, Analytical Sciences, 2003, 19,199
[12]Y. Lijour, J.Y. Calves and P. Saumagne, J. Chem. Soc. Faraday Trans. , 1987, 83 , 3283.
[13]F. Caruso, H. A. Rinia and D.N. Furlong, Langmuir 1996, 12, 2145.
[14]S.Z.Yao, Piezoelectric Chemistry and Biosensors, 1997, Hunan Normal University Press.
[15]T. Nomura, F. Tanaka, T.Yamada and H. Itoh, Anal.chim.Acta, 1991, 243, 273.
[16]T. Nomura, and T. Egawa, Analytica Chimica Acta, 1997, 339, 187
[17]D.Z.Shen, S.M.Peng, Q.Kang, Y.H.Xue and Fresenius J., Anal. Chem., 1998, 361, 424.
[18]T. Nomura, M. Kumagai, and A. Sato,, Analytica Chimica Acta, 1997, 343, 209
[19]S. Bao and T. Nomura, Analytical Sciences, 2002, 18, 881
[20]Q. Kang, X. Wu, Y.H. Xue, X.Y. Liu, D.Z. Shen, Sensors and Actuators B: Chemical 1999, 61, 68.
[21]J.T. Hu, D.L.Yang, Q.Kang, D.Z. Shen, Sensors and Actuators B: Chemical 2003,96, 390.
[22]D.Z. Shen, Q. Kang, X.L. Zhang, W. P. Li and Z. C. Liu, Mikrochicm.Acta 2002, 138, 89.
[23]P. Somasundaran and H.S.Hanna, Improved Oil Recovery by Surfactant and PolymerFlooding (D.O. Shah
and R.S. Schechter, Eds.), 1977, Academic Press, New York, 205.
[24]S. Partyka, S. Zaini, M. Lindeimer and B. Brun, Colloids Surf. 1984, 12, 255.
[25]D.Z.Shen, S. Lin, Q.Kang, L.H.Nie and S.Z. Yao, Analyst, 1993, 118, 1143.
[26]G.Sauerbrey, Z. Physik, 1959, 155, 206.
[27]F. G. Palmino., R.Denoyel and J.Rouquerol, J. Colloid Interface Sci. 1994, 165 ,82.
[28]P.Levitz, H.Van Damme and D. Keravis, J. Phys. Chem. 1984, 88, 2228.
[29]A. Gellan and C.H. Rochester, J. Chem. Soc. Faraday Trans., 1985, 81, 2235.
___________________
2004 Copyright ©, International Frequency Sensor Association (IFSA). All rights reserved.
(http://www.sensorsportal.com)
161