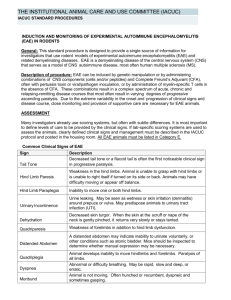
Induction of Experimental Allergic Encephalomyelitis (EAE)
advertisement

Induction of Experimental Allergic Encephalomyelitis (EAE) Policy No: 104.15 Revision No: 4 Effective Date: March 25, 2014 Category: Thomas Jefferson University Policies and Procedures This policy only applies to the induction and monitoring of EAE mouse models. It does not apply to drugs, treatments, surgeries, or any other materials or procedures that may be used in conjunction with EAE mice. Any modifications to the EAE policy by the PI must be scientifically justified by the investigator and approved by the IACUC. Introduction: Experimental Allergic Encephalomyelitis (EAE) is a demyelinating disease of the central nervous system and it serves as the animal model for Multiple Sclerosis (1, 2). EAE can assume an acute, chronic, or relapsing-remitting disease course that is dependent upon the method of induction and type of animal used. In general, EAE will assume a variable disease course that progressively results in escalating degrees of ascending animal paralysis. The resulting paralysis is debilitating, but not painful and most animals will show some degree of recovery even from advanced stages of EAE. Paralysis usually begins with a weakened tail, gradually followed by hind limb paralysis, and rarely front limb paralysis. EAE disease progression can be monitored with a scoring system that can differ between investigators but always starts with the normal condition and ends when the mice become moribund. Since EAE is a variable disease, there is no reliable way to predict whether an animal will recover. As a result, close monitoring is needed in this animal model. EAE can be induced with components of the central nervous system (3, 4) or peptides (5,7) and also via T cell transfer from one animal to another animal (8). Complete Freund's Adjuvant (CFA) must be used with the extracts or peptides and is often used in combination with pertussis toxin (9, 10). Pertussis toxin is thought to play a role in the breakdown of the blood-brain-barrier and may also increase interferon-У production (11). It is not possible to administer analgesics to lessen any pain that may be associated with the CFA injections, as most analgesics affect the immune response that is an essential component of the model (12, 13). This policy is intended for investigators using the EAE model, providing justifications and guidelines for the use of CFA, pertussis toxin, cell transfer, and monitoring of the animals. Guidelines: 1. CFA and Pertussis Toxin Guidelines EAE can be induced with components of the central nervous system or peptides mixed in CFA. a) CFA can be administered in 2-4 subcutaneous injections per site per day. b) Injection sites should be far apart. These sites should be near the axillary and inguinal lymph nodes (11). c) The concentration of CFA for EAE induction rages in the literature from 15 mg/ml (14, 15). In general, a total of volume 200 uL of emulsion containing a 1:1 ratio of antigen/CFA is administered to each mouse. Thus the total amount of CFA that can be administered to each mouse is 0.2 mg - 1.0 mg. The higher mycobacterial concentration is an accepted alteration to the Thomas Jefferson University (TJU) Complete Freund’s Adjuvant Guidelines (Policy 104.08). d) CFA can be administered on two days, but no more than two days without scientific justification. This is an accepted alteration to Thomas Jefferson University (TJU) Complete Freund’s Adjuvant Guidelines (Policy 104.08). e) The first CFA injection can be followed by Incomplete Freund’s Adjuvant (IFA), or with a combination of CFA, IFA, and/or pertussis toxin in place of the second day of CFA injections permitted in (c) above. f) The principal investigator (PI) must ensure monitoring of the animals post CFA injection for excessive pain, distress, or necrosis as described in Complete Freund’s Adjuvant Guidelines (Policy 104.08). Animals in such conditions will either be euthanized or the veterinary staff will be promptly consulted for advice. g) If pertussis toxin is used, an approval letter for its use in animals from the Lab Safety Committee must be submitted with the AUP form. Ranges of 200-500 ng pertussis toxin per injection are reported in the literature. Pertussis toxin may be administered iv or ip (11, 15). h) For induction of EAE by cell transfer, the recipient animal may be irradiated before cell transfer. Any transport of animals to the irradiator must follow TJU policy Movement and Transport of Research Animals (Policy 103.04). In the AUP, the PI must have approval from Radiation Safety Office (RSO) for irradiating animals (RSO Forms IBC13 and13A). 2. Biological Hazard Guidelines: Cells, Extracts, Peptides, Pertussis Toxin a) Extracts or cells used to induce EAE will be obtained from animals housed in the same TJU facility. There are several TJU mouse facilities; BLSB/Barrier, JAH/Barrier, JAH/Nonbarrier, etc. If this material is obtained from animals in a different facility, the Office of Animal Resources (OAR) must be consulted and pathogen testing may be required. b) Pertussis toxin must be rendered free of Bordetella bacteria either by filtration through a 0.2 or 0.1 micron filter or the manufacturer must certify that the preparation is Bordetella free. The PI should retain documentation from the manufacturer to this effect. Pertussis toxin is unlikely to be contaminated with eucaryotic viruses. c) Peptides should be sterilized by filtration or other means, unless sterility was guaranteed by the manufacturer, and stored in sterile containers. Chemically synthesized peptides are probably pathogen free, but aseptic technique must be maintained regarding storage and dilution. d) Preparation or culture of cells, preparation of extracts, and mixing of adjuvant with EAE inducers will be conducted in IBC certified BL2 facilities following BL2 practices. The mouse facilities at TJU are BL2 rated. 3. Scoring Systems and Monitoring Animal Health a) Scoring systems can differ in this model. An investigator's scoring system must be defined in the AUP application in reference to what each score means in terms of animal appearance. b) When animals start to experience hind limb paralysis, daily (including weekend) monitoring must be done from this time onward. c) When animals experience complete hind limb paralysis, food and water must be made easily accessible to the animals on the cage floor. This is an accepted exemption from Feeding Rodents on the Cage Floor (Policy 104.13). This can be done in the form of moistened food, semisolid food, gel packs (as used for animal shipping), etc. It has been noted that even in vent racks the animals are sometimes so debilitated that they lack the strength or persistence to obtain water, thus moisture must be made available to these mice in vent racks. The PI must indicate how food and water will be made accessible to the animals within the AUP. Consultation with the TJU veterinarians would be appropriate if in doubt regarding methods to supply food and water to debilitated animals. d) Any animal experiencing unusual pain or distress will be euthanized. Should less affected animals start fighting with affected animals, the animals will be separated. e) Death will not be an intentional endpoint. Moribund animals must be euthanized. Moribund is described as paralysis in all 4 limbs and trunk with labored, or reduced, breathing. If death is needed as an endpoint, the PI will provide an explanation with scientific justification in the protocol section of the AUP form. PI MUST STILL INDICATE THE FOLLOWING ON THE AUP FORM. 1) Means of inducing EAE, specifically, material (i.e. cell extract, peptide, cell transfer, etc.), route, dosage, volume, frequency and sites of EAE inducer injection and/or irradiation and cell transfer protocol. 2) Mouse strain(s) to be used. 3) Indicate whether this is an acute, chronic, or relapsing-remitting EAE model. 4) Define endpoints for the experiment. 5) Have Lab Safety and/or RSO approval for Pertussis toxin or irradiation procedure, respectively, or any other hazard (see above). Please attach approval letters to the AUP form. The use of hazardous agents must still be indicated in the appropriate section of the AUP. Use of other hazardous agents in conjunction with the EAE model may require other approval letters. 6) The PI will still need to address the search for alternatives in regard to Replacement, Reduction, and Refinement as this is a requirement that cannot be voided by the IACUC. For sections dealing with Justification and Use of CFA, Use of Biologicals, Monitoring, Pain and Distress, Use of Analgesics/Anesthesia to Lessen Pain and Distress, regarding the EAE model, it is permissible for the PI to state that they will adhere to the EAE Policy (104.15) without going into detail. THIS IS PROVIDED THAT THE PI IS NOT MAKING MODIFICATIONS TO THE POLICY. References: 1. Goverman J, B. T. 1996. Rodent models of experimental allergic encephalomyelitis applied to the study of multiple sclerosis. Lab Anim Sci. 46:482. 2. Paterson PY, D. E. 1981. Current perspectives of neuroimmunologic disease: multiple sclerosis and experimental allergic encephalomyelitis. Clin Immunol Rev. 1:581. 3. Levine, S., and R. Sowinski. 1973. Experimental allergic encephalomyelitis in inbred and outbred mice. J Immunol. 110:139. 4. Fritz, R. B., C. H. Chou, and D. E. McFarlin. 1983. Relapsing murine experimental allergic encephalomyelitis induced by myelin basic protein. J Immunol. 130:1024. 5. Tuohy, V. K., R. A. Sobel, and M. B. Lees. 1988. Myelin proteolipid proteininduced experimental allergic encephalomyelitis. Variations of disease expression in different strains of mice. J Immunol. 140:1868. 6. McFarlin DE, B. S., Kibler RF, McKneally S, Shapira R. 1973. Experimental allergic encephalomyelitis in the rat: response to encephalitogenic proteins and peptides. Science 179:478. 7. Linington C, B. T., Perry L, Weerth S, HinzeSelch D, Zhang Y, Lu HC, Lassmann H, Wekerle H. 1993. T cells specific for the myelin oligodendrocyte glycoprotein mediate an unusual autoimmune inflammatory response in the central nervous system. Eur J Immunol. 23:1364. 8. Yamamura T, N. T., Endoh M, Kunishita T, Tabira T. 1986. Passive transfer of experimental allergic encephalomyelitis induced by proteolipid apoprotein. J Neurol Sci. 76:269. 9. Lee JM, O. P. 1955. Simple method for enhancing development of acute disseminated encephalomyelitis in mice. Proc Soc Exp Biol Med. 89:263. 10. Kamradt T, S. P., Perkins DL, Gefter ML. 1991. Pertussis toxin prevents the induction of peripheral T cell anergy and enhances the T cell response to an encephalitogenic peptide of myelin basic protein. J Immunol. 147:3296. 11. Racke M, K. 2001. Experimental autoimmune enchepalomyelitis (EAE). Current Protocols in Neuroscience 2001:9.7.1. 12. Billiau A, M. P. 2001. Modes of action of Freund's adjuvants in experimental models of autoimmune diseases. J Leukoc Biol. 70:849. 13. Naiki M, T. Y., Kurimoto Y, Matsuoka T, Suehiro S, Imai Y, Osawa T, Gershwin ME. 1991. Neurotropin inhibits experimental allergic encephalomyelitis (EAE) in Lewis rats. Int J Immunopharmacol. 13:235. 14. Stromnes I, M. and J.M. Goverman. 2006. Passive induction of experimental allergic encephalomyelitis. Nature Protocols 1:1952. 15. Stromnes I, M. and J.M. Goverman. 2006. Active induction of experimental allergice encephalomyelitis. Nature Protocols 1:1810.