Chapters 7-10 Crossword Puzzle
advertisement

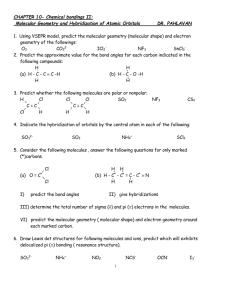
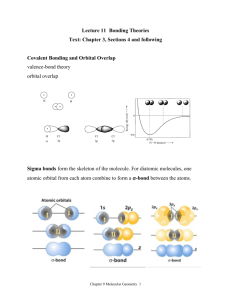
Chapters 7 - 10 Terms Chem 400 Nuss Name:________________________ Lab Section: 1 2 3 4 5 6 1 2 4 5 3 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 32 30 31 33 34 Across 3. orbitals of the same energy are said to be ____ 4. cycles per second 5. the maximum height of a wave 11. the molecular geometry for an AX6 molecule 13. the smallest possible division of light 17. the principle which states that orbitals of the same energy level each get 1 electron before any of them get a second electron is ____ ____ 18. the probability region for finding an electron based on the wave function psi 20. substances with unpaired electrons that are slightly attracted by a magnetic field 24. electrons in the interior of an atom that are not involved in bonding 25. substances that retain their magnetism after they are withdrawn from a magnetic field are called ____ 27. the net charge experienced by an electron based on shielding is the ___ nuclear charge 28. the distance between successive crests in a wave 30. the energy change when a gaseous atom looses an electron is the ___ energy 32. a covalent bond made by direct overlap of orbitals along the axis of the bond. 33. the number of waves passing a point in a given amount of time 34. in valence bond theory, atomic orbitals combine to form ___ orbitals during bonding Down 1. the molecular geometry for an AX5 molecule 2. substances without unpaired electrons that are slightly repelled by a magnetic field 3. a bond made up of one sigma and one pi bond 6. a covalent bond in which p orbitals share an electron pair above and below the bonded atoms 7. the ability of a covalently bonded atom to draw a pair of electrons towards itself 8. the molecular geometry for an AX3 molecule 9. the principle which states that no two electrons will share the same set of quantum numbers is the ____ ____ principle 10. the lowest energy level for an electron is known as the ___ state. 12. ions which share the same electronic configuration are said to be ____ 14. the quality of only having a discreet set of possible values 15. the molecular geometry for an AX4E2 molecule 16. electrons on the exterior of an atom that are available for bonding 19. the molecular geometry for an AX4 molecule 21. the energy change when a gaseous atom gains an electron is the electron ____ 22. the molecular geometry for an AX2E molecule 23. the molecular geometry for an AX4E molecule 26. the principle that states that you always fill orbitals of lowest energy first is the ____ principle 29. the molecular geometry for an AX2 molecule 31. a point of zero amplitude in a wave