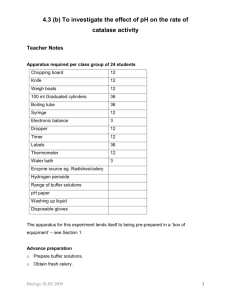
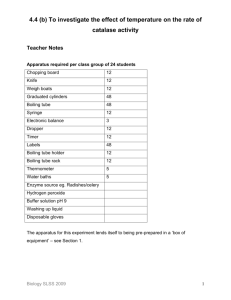
The Decomposition of Hydrogen Peroxide
advertisement

Elephant’s Toothpaste: The Decomposition of Hydrogen Peroxide Introduction Something decomposes when it breaks down into its component parts. Hydrogen peroxide is not a very stable compound, so it is always decomposing to water and oxygen. Under normal conditions, the decomposition goes very slowly. In this reaction, yeast catalyzes the decomposition, making the reaction go much more quickly. If you add a little dishwashing detergent, you get foam! If you add food coloring, you get colored foam! Safety Hydrogen peroxide can cause discomfort in the mucous membranes and the eyes therefore goggles must be worn when handling it. Do this experiment in the sink! Materials and Apparatus graduated cylinder 100 mL beaker glass rod 6% hydrogen peroxide Active yeast Warm water Liquid dish soap Food coloring - optional –but it does make a nice colour! Procedure 1. In a graduated cylinder, mix 10mL of liquid dish soap and 30 mL of 6% hydrogen peroxide solution. 2. Tip the graduated cylinder slightly and allow one drop of food colouring to drip down each side. 3. Place the graduated cylinder in the sink or a large basin. 4. In a 100mL beaker, mix one tsp of active yeast with 30mL of warm water stir with the glass rod until combined. 5. When you are ready, pour the yeast mixture into the graduated cylinder and watch the reaction! Observations How much foam is produced, and how quickly? Analysis 1. Does it matter if you use lukewarm water or cold water to activate the yeast? 2. What do you predict would happen if you added more or less soap? What's Happening?! Hydrogen peroxide (H2O2) decomposes into water and oxygen gas, but normally the reaction is so slow as to be imperceptible. 2H2O2(aq) 2H2O(l) + O2(g) What happens when you pour hydrogen peroxide onto a cut? It bubbles! That's because there is something in your bodily fluids that catalyzes the decomposition. A catalyst is a substance that speeds up a reaction, without being consumed itself. In this experiment we used a 6% hydrogen peroxide solution. The production of oxygen gas is made more noticeable by adding some dish soap, which makes the foam. The reaction is catalyzed by the active yeast added to the container. The yeast changes the mechanism, or pathway, by which the reaction occurs. The rapid production of bubbles of oxygen gas, along with the dish soap, quickly creates a large quantity of foam.