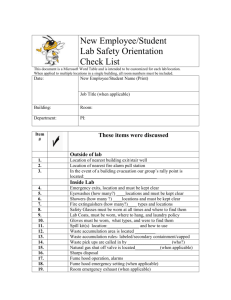
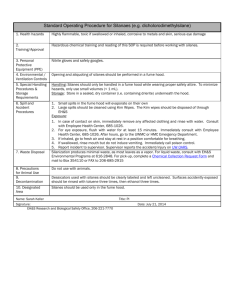
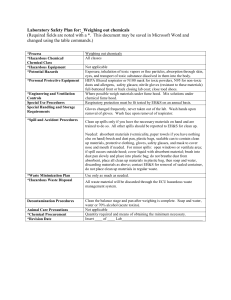
University of Minnesota Duluth Environmental Health & Safety Office
advertisement
UNIVERSITY OF MINNESOTA DULUTH Environmental Health & Safety Office Job-Task Hazard Analysis (JHA) Department: Chemistry & Biochemistry Task being performed: To synthesize biodegradable poly (lactide-co-glycolide) oligomers Date: September 9, 2009 Task to be performed by: Ann Polshyna Revised and Approved by: Mahjoub Labyad Title 29 of the Code of Federal Regulations (CFR) 1910.1450, Occupational Exposure to Hazardous Chemicals in Laboratories. Governing Standards In accordance with chemical MSDS and directions provided by lab supervisor. Tools and Equipment Used HPLC Equipment and Rotary Evaporator Analysis by: Alan Oyler Supervisor: Alan Oyler Task/steps sequence Potential or Associated Hazards Safe Operating Procedure Conducting reactions to synthesize biodegradable poly (lactide-co-glycolide) oligomers Glycolic acid and lactic acid [Corrosive materials, may cause severe burn if skin contact occurs and irritation if inhaled] see MSDS Gram quantities of glycolic and lactic acids are mixed together and heated 100-200 C in fume hood. Eye protection, gloves and lab coat should be worn Isolation of PLGA oligomers by preparative HPLC. Analytical HPLC analyses Acetonitrile and/or tetrahydrofuran are used as sample diluents and mobile phase solvents. [Highly toxic and flammable solvents may form peroxide upon exposure to air. May absorb through skin with same effect as inhalation. Fire and explosion hazards are of concern] (see below). Acetonitrile and/or tetrahydrofuran are used as sample diluents and mobile phase solvents. Rapamycin, BHT, and poly(lactide-co-glycolide) oligomers are the expected analytes. 1 Mobile phases should be prepared in fume hood. There are subsequently contained within the equipment. HPLC fractions are collected into 12mL test tubes in an automated fashion in a closed environment (by HPLC equipment). Appropriate fractions are combined and the solvent is removed on a rotary evaporator in a fume hood. Eye protection, gloves and lab coat should be worn Mobile phases should be prepared in fume hood. The resulting solutions are subsequently contained within the HPLC equipment. Eye protection, gloves and lab coat should be worn. Samples are weighed in a balance hood (with HEPA filter) and diluted with solvent in a fume hood. The resulting solutions are transferred to closed vials. Eye protection, gloves and lab coat should be worn Hazard Assessment: Potential Hazards Associated with the Procedure 1. Rotary Evaporator (rotovap) Working with a Rotovap presents a health hazard to employees depending on what solvent is used. Ensure that work with a rotovap is done in a well ventilated area, a fume hood in preference, to control vapor emissions into the lab space and minimize potential employee exposure to solvent vapors. If possible, avoid the use of water aspirators but use a diaphragm pump instead, to make sure fugitive emissions are kept to the minimum, as some solvent vapors do dissolve in the water used by the aspirator and are then released into the environment. Rotovap also presents a number of physical hazards such as implosions, explosions and the potential of fires. When vacuum is drawn within the rotovap’s glassware, there is risk of implosion. Therefore, all rotovap glassware must be inspected prior to each use to ensure it is free from cracks and imperfections. Use only heavy walled vacuum flasks and shatter-proof solvent collection bulbs. Also the use of ethereal solvents such as THF (see below) and other flammable materials in a rotovap presents a risk of fires and explosion hazards since these compounds form peroxides when exposed to air and light. When these solvent types are evaporated the residue is enriched with peroxides and violent explosions and fires may occur. Make sure only trained and authorized employees use the equipment. 2. Working with Tetrahydofuran (THF) CAS# 109-99-9 (see MSDS for further info.) THF (OSHA Permissible Exposure Limit (PEL) 200 ppm (TWA)) is a Chemical that forms explosive levels of peroxides when concentrated through distillation, evaporation or when exposed to air after opening. It can become extremely sensitive to thermal or mechanical shock and may explode violently. Peroxides are formed through a spontaneous reaction with oxygen. Simply opening the container can initiate peroxide formation, while light and heat can act to accelerate the process. Unless these materials are properly handled they can pose a serious safety hazard to laboratory personnel and become a difficult disposal problem for the UMD Environmental Health and Safety Office. Manufacturers usually add an inhibitor such as butylated hydroxyl toluene (BHT) to the peroxide forming chemical to counter the peroxide formation by scavenging oxygen in the solvent. Distilling or evaporating the solvent completely exhausts the added inhibitors, increases solvent exposure to oxygen in the air and can make the solvent highly rich in peroxides. Extreme caution must be taken to handle rotovap solvents when removing or disposing of them. THF, a class B peroxide former, must be dated when opened and should not be used if older than 12 months after opening, see (http://www.d.umn.edu/ehso/waste_management/special.html#peroxide) 3. Working with Acetonitrile CAS# 75-05-8 (see MSDS for further Info.) Acetonitrile, OSHA Permissible Exposure Limit (PEL) 40 ppm TWA over 8 hours, is a highly toxic solvent, with very poor warning properties (employees maybe exposed to it without notice). It readily absorbs through the skin and the effect of skin absorption is similar to that of inhalation (see MSDS for exposure symptoms). Work only in the fume hood when using this solvent. Working with this material in a rotovap presents both health and physical hazards. 4. Review MSDS for other chemicals listed in this SOP. 2 Required Personal Protective Clothing The following must be used at all time when conducting this research: Wear a lab coat, long pants and close toed shoes (No shorts, Skirts or Sandals). Use chemical splash goggles that meet ANSI Z87 requirements, when transferring acids or when working with the Rotovap of eye protection For hand protection, use (8ml) Nitrile gloves if accidental contact is expected, double gloves if necessary, if working with, or transferring large amounts of acetonitrile is required, it is recommended to use heavy weight butyl rubber or polyvinyl acetate (PVA) gloves. Hazard Control and Prudent Work Practices Ensure work is done in the fume hood. Verify that fume hood is operating properly before each use. EHS will make sure fume hood face velocity is between 90cfm and 110cfm. The actual fume hood Face velocity is at 147cfm, the room is under negative (15 cfm) pressure. Last checked September 15, 2009. Ensure fume hood sash is at 18 inch mark or below when being used and closed when access is not necessary or when highly toxic or reactive chemicals are in use in the hood. If for some reason work with organic solvents is done outside of the fume hood, exposure monitoring may be required to ensure chemical vapors are kept to the minimum and do not exceed permissible exposure limits (PEL). All general safe Lab work practices should be followed, including washing hands immediately after removing PPE, no eating, drinking, or chewing gum. Waste Disposal Follow guidelines found at: http://www.d.umn.edu/ehso/waste_management/stepbystep.html Collect waste in compatible containers Empty rotovap’s solvent flasks and turn off the heating bath immediately after each use. Transfer chemicals and chemical waste in the fume hood only. Ensure waste containers are tightly closed when not in use to prevent vapor emissions. Ensure waste containers are labeled correctly and bear the wording “Hazardous Waste”. Hazard Assessment and PPE Certification: This is to certify that the employee named below has reviewed the above information, understands the hazards associated with the procedure and is capable to use the assigned personal protective equipment PPE correctly. Employee Name: __________________________________________ Supervisor’s Name: _______________________________________ Date: ____________________________ 3 Signature: _____________________________________