ME 354 Tutorial #2 – Availability
advertisement

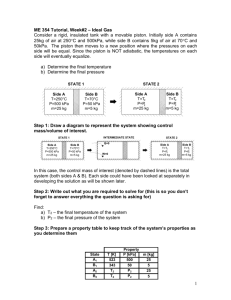
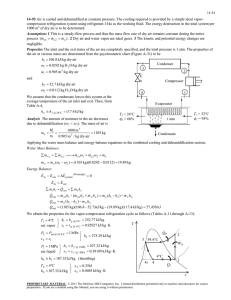
ME 354 Tutorial, Week#3 – Exergy: Control Mass Analysis An insulated piston-cylinder device contains 2L of saturated liquid water at a pressure of 150kPa – which is constant throughout the process. An electric resistance heater inside the cylinder is turned on, and electrical work is done on the water in the amount of 2200kJ. Assuming the surroundings to be at 25C and 100kPa, determine: a) The minimum work with which this process could be accomplished and b) The exergy destroyed during the process Step 1: Draw a diagram to represent the system (show control mass of interest) The control mass boundary encloses the water in the cylinder State1 Intermediate State Surroundings T0 =25°C P0 =100 kPa Wb Sat. Liquid H 2O P1 =150 kPa V1 =2L State 2 Surroundings T0 =25°C P0 =100 kPa Q=0 H20 P2 =150 kPa We = 2200 kJ Step 2: Property table State T [°C] 1 (sat liq) 2 P [kPa] 150 150 Property h [kJ/kg] s [kJ/kg*K] v [m3/kg] V [m3] 0.002 Step 3: Assumptions Assumptions: 1) Insulated piston-cylinder (Q=0) 2) KE, PE 0 3) Compression/expansion processes are in quasi-equilibrium Step 4: Calculations (usually start by writing First and Second Laws) Part a) We can determine the properties at state 1 using the information given in the problem (saturated liquid H2O at P = 150 kPa) with Table A-5. From Table A-5 s1 = sf@150kPa = 1.4336 [kJ/kg*K] v1 = vf@150kPa = 0.001053 [m3] h1 = hf@150kPa = 467.11[kJ/kg] From the specific volume at state 1, v1, and the volume of state 1, V1, we can determine the mass of H20 in the cylinder. 1 mH 2O V 1 v1 0.002 m3 1.9kg m3 0.001053 kg We still must determine the properties at state 2 (we DO know the pressure is 150 kPa because this is a constant pressure process). We can write an energy balance on the system to link state 2 to state 1, as shown in Eq1. E1 We Wb E2 (Eq1) Using the assumption KE, PE 0, we and noting that W b = P(V2-V1), Eq1 can be re-expressed as Eq2. U 2 U1 We P(V2 V1 ) (Eq2) Eq2 can be rearranged into the form presented in Eq3. U 2 PV2 U1 PV1 We (Eq3) Recognizing that U+PV = H, and that H = mH20h we can rewrite Eq3 as Eq4. mH 2O h2 h1 We (Eq4) Isolating h2 in Eq4, we can determine h2. h2 kJ kJ We 2200kJ h1 467.11 1625 mH 2O 1.9kg kg kg Using h2 = 1625 kJ/kg and P2 = 150kPa with Table A-5, we find that we are in the vapour dome, in between the saturated liquid and vapour states on the 150 isobar line. We can determine the properties at state 2 by first finding the quality, and then using this to interpolate in Table A-5. x h2 h f hg h f 1625 467.11 0.52 2693.6 467.11 s2= sf@150kPa + xsfg@150kPa = 1.4336 + 0.52(5.7897) = 4.44 [kJ/kg*K] v2= vf@150kPa + x(vg@150kPa -vf@150kPa) = 0.001053 + 0.52(1.1582) = 0.6033 [m3] 2 The minimum work that needs to be provided to accomplish this process represents the reversible work input (Sgen = 0 Xdestroyed = 0). We can use the general relation for reversible work as expressed in Eq5. Wmin Wrev (U 2 U1 ) T0 (S 2 S1 ) P0 (V2 V1 ) (Eq5) Substituting Eq2 into Eq5 for internal energy and bringing the m H2O outside the bracket to convert the properties to specific properties, Eq5 can be written as Eq6. (Eq6) Wmin We mH 2O P P0 v2 v1 T0 (s 2 s1 ) Substituting the known property values into Eq6, we can calculate the minimum work input. kJ kJ Wmin 2200[kJ ] 1.9[kg](150 100)(0.6033 0.001053) (298)( 4.44 1.4336) kg kg = 440.6 kJ Answer a) Part b) The exergy destroyed can be calculated from the expression Xdestroyed = T0Sgen. To find the entropy generated during the process we can perform an entropy balance on the system as shown in Eq7. S in S out S gen S system (Eq7) For a closed system, entropy can only be carried across the system boundary by heat transfer. Since the system is insulated, Sin = 0 and Sout = 0. The change in the system entropy is the difference in entropy between state 2 and state 1 i.e. Ssystem = mH2O(s2-s1). Using the above information with Eq7, the Xdestroyed (Irreversibility) can be determined. kJ X destroyed T0 S gen T0 mH 2O ( s 2 s1 ) (298[ K ])(1.9[kg])( 4.44 1.4336) kg K = 1702.2 kJ Answer b) Step 5: Concluding Statement and Remarks The minimum work with which this process could be accomplished is 440.6kJ. The amount of exergy destroyed during this process is 1702.2 kJ. 3