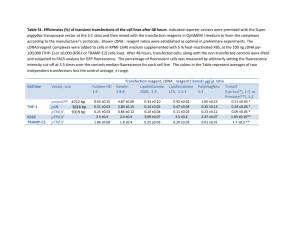
Supplementary Data - Word file
advertisement

Supplementary material Materials and Methods Plant Material The original seeds from the T-DNA insertion lines in Columbia (Col-0) background At1g68830 (SALK 073254) and At5g01920 (SALK 060869 and SALK 064913) were germinated and selected on kanamycin-containing plates (a selectable marker in the TDNA region) before they were transplanted in soil. Plants from the T3 kanamycinresistant generation were selfed, and the progeny were subjected to DNA extraction and PCR analysis. For STN7 the oligos used were At073254-Fw (CAAACAATTAAGTTTGCACC), At073254-R (TGAGGACTCATGTTTTGTGTC), At060869-Fw (GGGCCACTATTGAGATGATTG), At064913-R (CGTCATCAACAAGAGTAGAATTC) and T-DNA Left Border (ATCAGCTGTTGCCCGTC). For STN8 the oligos GGGCCACTATTGAGATGATTG and CGTCATCAACAAGAGTAGAATTC were used. Plants homozygous for the TDNA insertion were identified based on the PCR analysis. The homozygous stn7 was backcrossed to the wild type and the F1 progeny were selfed. Homozygous stn7 plants were recovered and used for the experiments. Double mutants were obtained by crossing homozygous stn7 with homozygous stn8 and the progeny were analysed by PCR. Complementation with Stn7 and Stn7-HA 1 A 7253-bp STN7genomic fragment containing a region encompassing 4056 bp upstream of the start codon and 379 bp downstream of the stop codon was excised by digesting the BAC clone T6L1 with XhoI. The fragment was inserted into the pSK vector at the XhoI site. This XhoI fragment was cloned in the unique SalI restriction site of the binary plant transformation vector pPZP312, derived from a pPZP vector with a Basta selectable gene under the control of the 5’ mas promoter and the 3’ mas terminator 1 giving rise to plasmid pPZP-At1g. This plasmid was then introduced into the A. tumefaciens strain GV3101. Transformation of Arabidopsis plants was achieved by the floral-dip method using Silwet L-77 2. Seeds harvested from transformed plants were germinated on ½ MS medium (Sigma) containing 10 mg L-1 of Basta and 0.8% agar for 2 weeks, and Bastaresistant Arabidopsis plants were selected. Seedlings were then transplanted to peat and tested for the presence of STN7. A full-length cDNA clone of STN7 (accession number AY094447) was obtained from the Riken Institute. To remove a point mutation in this full-length cDNA we also used an EST from the STN7 gene (accession number AV552222) from the Kasuza resource center. To introduce the 3' end of STN7 cDNA in frame with the triple HA tag in the binary vector pCF399, the STN7 cDNA was first subcloned in a sense orientation in Xho1 and Sac1 restriction sites of pSK producing the pSK-AT1c plasmid. The 3' end of the Stn7 ORF was then engineered by PCR to introduce a Sal1 restriction site in frame immediately before the stop codon using oligonucleotides At075552R1 (TGCTCTTGCATCAGCACTTAG) and At1cSalSac-R (GCGAGCTCCCGTCGACCTCCTCTCTGGGGATCCATCGG) producing plasmid pSK-At1cSalSac. The mutagenized cDNA was then excised by Xho1 and Sal1 and 2 ligated in frame with the HA tag in the Sal1 site of plasmid pCF399 derived from the pPZP312 vector containing a 35S promoter and a Rbcs terminator, yielding plasmid pCF399-At1cHA. The 2.1 kb PpuM1 fragment of this plasmid, comprising the STN7 3' end, a 3xHA tag and the RbcS terminator, was then introduced in the pPZP-At1g plasmid producing a chimeric STN7 gene/ HA-tagged cDNA construct named pPZP-At1cgHA. In order to reinsert all introns (except intron 9) a 2.3 kb Nco1 / BglII fragment was excised from STN7 gene and reintroduced in pPZP-At1cgHA yielding plasmid pPZPMidiAT1HA. DNA sequencing was performed by Fasteris (Geneva). RNA Analysis Small-scale extraction of RNA from one or two leaves from 4-week-old plants was performed with the TRIzol reagent (Gibco BRL, Gaithersburg, MD) according to the manufacturer’s instructions. Reverse transcription reactions were performed in 20 μl with 1 μg of total RNA using 1 μl of Extand reverse transcriptase (Roche) and 10 pmol of oligo-dT as primer according to the manufacturer’s instructions (Roche) or with M-MLV Reverse Transcriptase RNase H Minus Point Mutant (Promega) using 2 g of total RNA extracted from leaves of 2 week-old plants and DNase-treated according to the manufacturer instructions. PCR were performed with DyNAzyme EXT DNA polymerase (Finnzyme) using 0.2 nM of sense and antisense primers At1fus4/5fw (CCTTATAATGTAGAAACTATCATC) and At1fus8/9rw (GGAATGCCATTTGAAG) for amplification of STN7 cDNA; ex23STN8 (TTCGAGGGAGACCGTG) and ex34STN8 (ACTGTTCAACTGCCAGAG) and AtUbiq1 3 (GATCTTTGCCGGAAAACAATTGGAGGATGGT) and AtUbiq2 (CGACTTGTCATTAGAAAGAAAGAGATAACAGG) as loading control. Localization of Stn7 Chloroplasts were isolated from four plants at the rosette stage grown under short day conditions (8h light, 16h dark) maintained 24h in darkness at 4°C just before harvesting. Leaves were broken in CIB buffer (0.45 M sorbitol, 50 mM Hepes pH 7.8, 10 mM EDTA, 1 mg mL-1 BSA, 2.5 mM MgCl2 and Sigma protease inhibitor cocktail (P8849 from Sigma)) in a Waring blender. The macerate was filtered through two layers of Miracloth, debris were eliminated by centrifugation for 5 s at 1000 g, and the material was concentrated by centrifugation for 7 min at 1000 g, resuspended in 1 ml of CIB and layered on a discontinuous 40%/80% Percoll gradient. Intact chloroplasts were then isolated at the interface of the two layers after centrifugation for 15 min at 7000 g. Pure chloroplasts isolated after three Percoll gradient centrifugations were washed twice in HMS buffer (0.33 M Sorbitol, 50 mM Hepes pH 7.8, 2.5 mM MgCl2 and Sigma protease inhibitor cocktail), centrifuged 5 min at 1000 g and resuspended in 0.1 ml HMS. For soluble/insoluble fractionation, chloroplasts were broken by osmotic lysis and sonication in lysis buffer (20 mM Hepes pH 7.8.5 mM EDTA, 0.1 M NaCl) and ultracentrifugation for 30 min at 100’000 g. Total extracts were obtained by grinding and vortexing one Arabidopsis leave in breaking buffer (50 mM Tris pH 8, 10 mM EDTA and Sigma protease inhibitor cocktail). Debris were eliminated by centrifugation for 5 s at 10 000 g and the 4 supernatant was either used directly, or used to separate soluble and insoluble material by centrifugation for 30 min at 100 000 g. Transient expression of GFP-STN7 constructs in A. thaliana protoplasts The full length STN7 and its N-terminal coding regions were amplified by PCR using primer FwNcoATGstn7 (TCCGCCATGGCTACAATATC) and, respectively, primers RwNcosstopstn7ala (GTATCCATGGCCTCCTCTCTGGGGATCCATCGG) and RwNcoPepTransstn7 (GATACCATGGCAGTGATCGTTAAACCATTAG). The PCR fragments were subcloned in PCRII plasmid by T/A cloning for sequencing and subsequently inserted in frame with the EGFP in the NcoI restriction site of pCL60 (gift of F. Kessler, University of Neuchâtel, Switzerland), which harbors a CaMV 35S promoter and a Tnos terminator. Preparation and transfection of protoplasts from A. thaliana was performed as described 3,4. Protoplasts were resuspended in TE supplemented with Sigma protease inhibitor cocktail and broken by repeated pipetting through a 25G1 needle. Based on chlorophyll contents, equal amounts of extracts were further fractionated between soluble (S1) and insoluble fractions by ultracentrifugation 30 min at 100 000 g. Proteins weakly associated to the membranes were recovered by resuspending the pellet in 0.1 M Na2CO3. After 30 min the extract was centrifuged for 30 min at 100 000 g. The two supernatants (S1 and S2) and the pellet (P) were analysed on a 12.5 % SDS PAGE. 5 Protein extraction and immunoblot analysis Thylakoid membranes were prepared as described 5. The chlorophyll content of the protein samples was determined as described 6 and all the samples were loaded on a chlorophyll basis. All the thylakoid membrane proteins were resolved by SDS-PAGE in 12 or 15 % acrylamide gels. Immunoblotting was performed by incubation with different polyclonal antibodies and detection with the ECL system (Amersham Biosciences, Inc.). State 1-state 2 transitions An intact leaf from 30 min dark-adapted plants was fixed to a light guide, and maximum fluorescence yield (Fm) was measured during exposure to a saturing flash (0.8 s, 6000 μmol m-2 s-1) using a pulse amplitude modulation fluorometer (Hansatech Ltd., King’s Lynn, England). The leaf was kept in the dark for 5 min and a first Fm measurement was performed. Then the leaf was illuminated for 15 min with 80 μmol m-2 s-1 blue light (PSII light) from high intensity light source LS2 (Hansatech Ltd., King’s Lynn, England) equipped with a Scott BG38 filter. Subsequently a far-red light (PSI light) provided by a LED source with a peak wavelength at 735 nm was switched on (Hansatech Ltd., King’s Lynn, England), and after 15 min the maximal fluorescence yield in state 1 (Fm1) was determined. The far-red light was switched off and the maximum fluorescence yield (Fm2) was measured after 15 min of blue light excitation. The relative change in fluorescence was calculated as ΔF= ((Fm1-Fm2)/Fm1) x 100. 6 Low temperature emission spectra Chlorophyll fluorescence emission spectra of thylakoid membrane suspensions were recorded in liquid nitrogen (77 K). Thylakoid membranes were prepared as described 7, and the chlorophyll concentration of the membrane preparations was determined in 80 % acetone 6. Chlorophyll fluorescence emission spectra were recorded with a Jasco FP-750 spectrofluorimeter using thylakoid preparations diluted to an equal chlorophyll concentration (5 μg/ mL) in a matrix consisting of ice and quartz particles 8 in 20 mM HEPES/NaOH, 3 mM MgCl2, pH 7.5 buffer. Excitation light was at 435 nm (10 nm slit width), and emission was detected from 650 to 800 nm (5 nm slit width). Thylakoid membranes (400 μL) were frozen in liquid nitrogen. Chlorophyll fluorescence emission spectra exhibit one peak at 685 nm due to chlorophyll a associated with PSII and one at 735 nm from chlorophyll a molecules associated principally with PSI. All spectra were normalized at 685 nm. Plant growth under controlled conditions of light In order to limit variations in photosynthetic activity due to stomatal regulation and increase the reproducibility of gas exchange measurements, experiments were carried out under non-photorespiratory conditions (1.2 % O2, 750 µmol CO2 L-1 air). The gas flow rate at the leaf level was 250 mol air s-1 and the temperature was 25°C. Air humidity was controlled by a dew point generator (LI-COR Biosciences, LI-610) set at 17°C. Gas exchange and chlorophyll fluorescence were measured at different actinic light intensities supplied with light emitting diodes (90 % red light, 10 % blue light) following a 20 min equilibration period under a light intensity of 150 µmol m-2 s-1. Fluorescence levels, Fs 7 (stationary fluorescence level in the light), Fm’ (maximal fluorescence in the light measured following a saturating pulse) and F0’ (basal fluorescence of light-adapted leaves, recorded after rapid reoxidation of the PQ pool using far-red light), were used to calculate non-photochemical quenching (NPQ = 1-Fm’/Fm) and photochemical quenching (qP = (Fm’-Fs)/(Fm’-F0’)) under different light intensities 9. References 1. 2. 3. 4. 5. 6. 7. 8. 9. Hajdukiewicz, P., Svab, Z. & Maliga, P. The small, versatile pPZP family of Agrobacterium binary vectors for plant transformation. Plant Molecular Biology 25, 989-994 (1999) Clough, S. J. & Bent, A. F. Floral dip: a simplified method for Agrobacteriummediated transformation of Arabidopsis thaliana. Plant J 16, 735-43. (1998). Jin, J. B. et al. A New Dynamin-Like Protein, ADL6, Is Involved in Trafficking from the trans-Golgi Network to the Central Vacuole in Arabidopsis. Plant Cell 13, 1511-1526 (2001) Bauer, J. et al. Essential role of the G-domain in targeting of the protein import receptor atToc159 to the chloroplast outer membrane. J. Cell Biol. 159, 845-854 (2002) Havaux, M., Dall'Osto, L., Cuine, S., Giuliano, G. & Bassi, R. The effect of zeaxanthin as the only xanthophyll on the structure and function of the photosynthetic apparatus in Arabidopsis thaliana. J Biol Chem 279, 13878-88. (2004) Porra, R. J., Thompson, W. A. & Kriedemann, P. E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophyll a and b with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta 975, 384394 (1989) Robinson, H. H. & Yocum, C. F. Cyclic photophosphorylation reactions catalyzed by ferredoxin, methyl viologen and anthraquinone sulfonate. Use of photochemical reactions to optimize redox poising. Biochim Biophys Acta 590, 97-106 (1980) Weis, E. Chlorophyll fluorescence at 77K in intact leaves: Characterization of a technique to eliminate artifacts related to self-absorption. Photosynthesis research 6, 73-86 (1985) Genty, B., Briantais, J. M. & Baker, N. R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 990, 87-92 (1989) 8 Figure legends Figure S1. Locations of T-DNA insertions in STN7 (At1g68830) and STN8 (At5g01020) and RT-PCR of STN7 and STN8 transcripts in the wild-type (Col-0), stn7, stn8 and in the rescued stn7-1R, stn7-4R, stn8-13 and stn8-69 lines. The identity of the fragments obtained by RT-PCR was confirmed by sequencing. Ubiquitin RNA (Ub) was used as standard. Figure S2. The STN7 kinase is localized in chloroplast membranes. A. Total leaf extracts (T) were separated into a soluble (S) and membrane fraction (M). Isolated chloroplasts (Tc) were broken and separated in soluble (Sc) and membrane (Mc) fractions. The chloroplast membrane fraction was treated 5 min with 40 U of calf intestinal phosphatase (CIP). Proteins from the different fractions were separated by SDS-PAGE and immunoblotted with antisera against HA, PsaA (PSI membrane protein), phosphoribulose kinase (PRK, a soluble chloroplast protein) and DET3, a cytosolic protein. The latter immunoblot shows that the chloroplast fraction is not contaminated with cytosolic proteins. Note that the signal of STN7 is not enriched in the chloroplast fraction because of the instability of STN7 during the lengthy chloroplast isolation. PsaA is also phosphorylated. B. Arabidopsis protoplasts were transformed with STN7-GFP, STN71-88GFP containing the 88 N-terminal amino acids of STN7, and GFP. GFP and chlorophyll (Chl) fluorescence were observed by confocal laser scanning microscopy using a Leica DM IRBE microscope and a Leica TCS SP laser. M, merge of GFP and chlorophyll fluorescence. T, transmission microscopy of transformed protoplasts. C. Immunoblotting 9 of protoplast fractions. Protoplasts were broken and separated in supernatant (S1) and total membrane fraction. The latter was extracted with carbonate and separated in pellet (P) and supernatant (S2). Proteins were fractionated by PAGE and detected with GFP antibodies. The sizes of the detected bands were as expected. Figure S3. Immunoblot analysis of proteins of the photosynthetic apparatus. Different loadings of thylakoid proteins from the Col-0 and stn7 lines were separated by SDSPAGE and immunoblotted with antiserum of D1 (PSII), LHCII, PsaA (PSI), LHCI, Rieske protein (cytochrome b6f complex)CF1 (ATP synthase) and TAK kinase. Signals were visualized with ECL. 10