Skeletal Muscle Contraction
advertisement
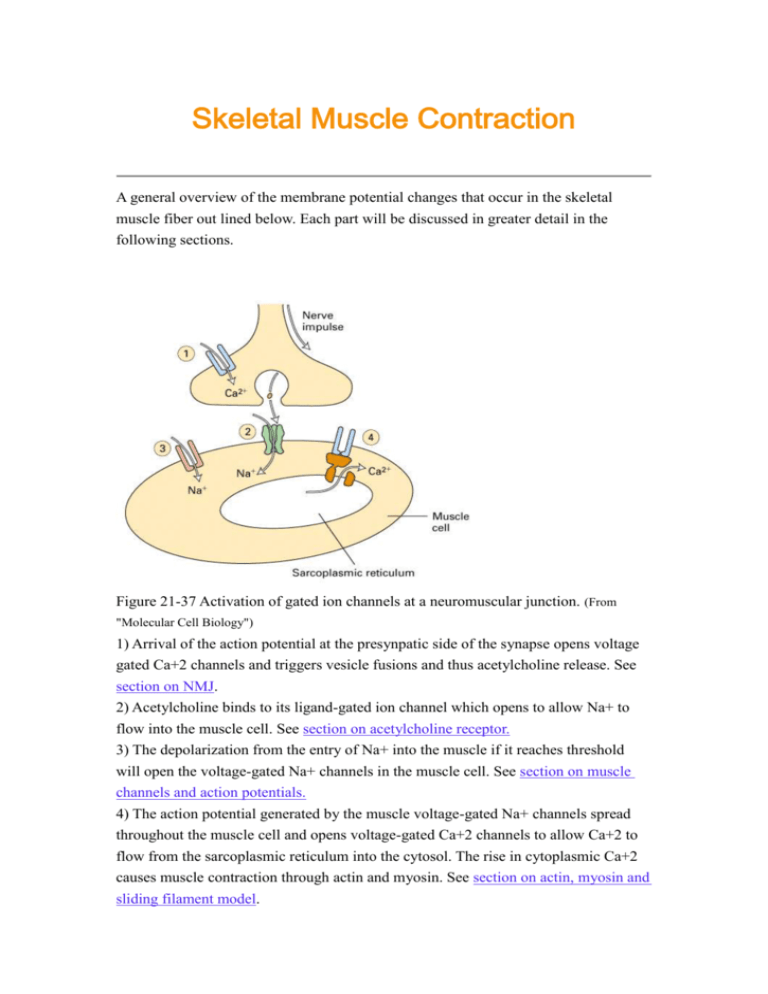
Skeletal Muscle Contraction A general overview of the membrane potential changes that occur in the skeletal muscle fiber out lined below. Each part will be discussed in greater detail in the following sections. Figure 21-37 Activation of gated ion channels at a neuromuscular junction. (From "Molecular Cell Biology") 1) Arrival of the action potential at the presynpatic side of the synapse opens voltage gated Ca+2 channels and triggers vesicle fusions and thus acetylcholine release. See section on NMJ. 2) Acetylcholine binds to its ligand-gated ion channel which opens to allow Na+ to flow into the muscle cell. See section on acetylcholine receptor. 3) The depolarization from the entry of Na+ into the muscle if it reaches threshold will open the voltage-gated Na+ channels in the muscle cell. See section on muscle channels and action potentials. 4) The action potential generated by the muscle voltage-gated Na+ channels spread throughout the muscle cell and opens voltage-gated Ca+2 channels to allow Ca+2 to flow from the sarcoplasmic reticulum into the cytosol. The rise in cytoplasmic Ca+2 causes muscle contraction through actin and myosin. See section on actin, myosin and sliding filament model. Skeletal muscle (striated muscle) Skeletal muscle cells are one of the largest cells in the body. They are multinucleate formed by the fusion of myoblasts. Diameters range from 50 to 150 microns with lengths ranging from mm to cm. To be effective the whole muscle fiber develops tension at the same time and of course to generate force the muscle fiber is anchored. Mucle fibers contract in response to an electrical signal, depolarization. The signal is generated at the synpase (the neuromuscular junction) and propogated through an action potential via the muscle fiber membrane. The membrane of the cell has specialized invaginations called Transverse-tubules (T-tubules) that enter into the cell (at every 1-2 microns). Thus the action potential can be rapidly transmitted deep into the interior of the cell resulting a delay of only 3-5 msec between the depolarization at the synpase and the first muscle fiber tension. The T-tubule network is so extensive that 50-80% of the plasma membrane is in the T-tubules. Figure 18-31A. A model of a muscle fiber composed of six myofibrils. (From "Molecular Cell Biology") The plasma membrane containing the ion channels sends invaginations (called T tubules) into the myofibers at the point of the Z disks. The T tubule contacts the sarcoplasmic reticulum forming a structure called a triad. At this point in the plasma member is a voltage-gated Ca+2 channel (dihydropyridine receptor) which is associated with the Ca+2 release channel (ryanodine receptor) in the sarcoplamic recticulum membrane. Vertebrate skeletal muscle is striated given the arrangement of action and myosin in each sacromere. We will discuss at length the function of the muscle fiber from the generation of the depolarizing endplate potential to the triggering of the myosin/actin interactions to generate contraction. Neuromuscular junction Figure 21-33. High magnification image from a frog neuromuscular junction. (From "Molecular Cell Biology") (Figure from "Cell Physiology Source Book") Each muscle fiber is innervated by a motorneuron. One motorneuron can innervate one or multiple fibers. Each motorneuron plus its complement of muscle fibers is called a motor unit as well all contract in concert. The synpase between the motorneuron and the muscle fiber is called a neuromuscular junction (NMJ) and is a unique type of synpase. The motor nerve terminal branches run in shallow grooves on surface of muscle. The nerve terminal contains many mitochondria and vesicles which can be seen lined up in double rows along a region of dense material attached to presynaptic membrane => active zone. The dense material has been postulated to include the voltage-gated Ca+ channels. The vesicles of the NMJ have very high concentrations of neurotrasmitter (2,000 to 10,000 molecules of acetylcholine per vesicle). This is not a subtle synapse that generates a small epsp. Rather the function of the NMJ is to generate a contraction in the muscle fiber there an excess of neurotransmitter is released to ensure that the resulting post-synaptic depolarization is strong enough to generate an action potential. The average post-synaptic potential in a muscle fiber is a depolarization of 40 mV. The post-synaptic depolarization is always much greater than threshold for an action potential (rest is -80 to -70 mV and threshold for an action potential is -60 to -50 mV on average). This is due in part to the release of a lot of neurotransmitter at the NMJ and a high concentration of receptors on the post-synaptic side. The synpatic cleft of the NMJ is very structured, containing an extensive basal lamina which contains proteins that are only found at the NMJ. For instance the acetylcholine esterase proteins are physically bound to the basal lamina to keep the enzyme localized to the synpase. On the muscle side the post-synaatic membrane (called the motor endplate) has many postjunctional folds which are particular to skeletal muscle synapses. These fold are full of the neurotransmitter receptor which is: acetylcholine is the neurotransmitter for the mammalian NMJ glutamate is the neurotransmitter for the insect NMJ Acetylcholine receptor Studies to look at the numbers of acetylcholine receptors found ~15,000 receptors per micron2. To do these studies researchers used a toxin that is high specific for Achreceptors called alpha-bungarotoxin. Alpha-bungarotoxin is another example of a snake venom used by the predator to kill its prey. Researchers use the toxin by labelling the toxin with either 125I or a fluorescent label, appling the toxin to a NMJ preparation and then assaying where the receptors are by following the radio label or the fluorescence label. These types of studies showed a very high concentration of receptors in the synpase but a complete absence of receptors away from the synapse. The molecular nature of the acetylcholine receptor is well studied as you might except these proteins are highly conserved between all animals. The acetylcholine receptor is made up of five subunits (all have been cloned): 2 alpha, 1 beta, 1 gamma and 1 delta subunit. (Figure from "Cell Physiology Source Book") Figure 21-38A. Model of the structured of the ligand gated acetylcholine receptor. (From "Molecular Cell Biology") The receptor is made up of 4 different transmembrane proteins one of which (the alpha subunit) is repeated to give 5 subunits to create the ion channel. Acetylcholine binds to the alpha subunit and thus it takes two molecules of acetylcholine to open the channel. One acetylcholine receptor opens and allow 1.5 x 104 Na+ ions/msec of open time. The channel opens on average 1 msec. Therefore one open channel causes a depolarization of about 0.3 V. From other studies it has been shown that the amount of neurotransmitter contained in one vesicle causes an post-synaptic potential of ~ 1 mV. Therefore 1 vesicle contains enough neurotransmitter to open ~3000 receptors and because two molecules of Ach is needed to open one receptor there must be a minimum of ~6,000 molecules Ach per vesicle. If the average depolarization generated at a NMJ of a muscle fiber is 40 mV then there must be at least 40 vesicles released and in the order of 133,000 receptors activated at the NMJ. (Figure from "Cell Physiology Source Book") The postsynaptic response of the muscle fiber is the same as an epsp in a neuron except that it is called an endplate potential (epp). The influx of Na+ through the receptor causes a depolarization that is normally sufficient to open the voltage-gated Na+ channels found in the muscle fiber. To record and analyze the endplate potential reseachers first have to remove the influence of the voltage-gated Na+ channels which of course generate action potentials that would mask any of the smaller membrane potentials made by the acetylcholine receptors. To do this researchers simply add TTX which is a highly specific toxin that blocks voltage-gated Na+ channels. Now in the absence of action potentials the membrane potentials generated by the acetylcholine receptor can be measured. The above figure shows the recorded endplate potentials from a muscle fiber with a recording electrode at the NMJ (0) and 1, 2, 3 or 4 mm away. The amplitude of the epp is reduced the further away from the initial depolarization. This is precisely the same mechanism that we saw in the dendrites of neurons and the same sources of current loss (i.e. cable properties, Cm, Rm and Ri) are in effect here. The length constant can be determined from these types of experiments and on average the length contant is ~ 1 mm. Remember though the muscle can be mm to cm in length and needs to contract rapidly and smoothly i.e. across its entire surface not just in one spot. Thus the presence of voltage-gated Na+ channels are essential to propogate the signal rapidly without loss of signal to the entire muscle surface. The presence of the T-tubules delivers the action potential deep into the muscle interior. Finally the NMJ is usually centred in the middle of the muscle fiber resulting in action potentials following in two directions away from the NMJ. Striated muscle channels and action potentials (Figure from "Cell Physiology Source Book") The skeletal muscle has a similar array of voltage-gated ion channels, transporters and pumps as the neurons we discussed in previous sections. Pumps and transporters - these were discussed in the previous lectures (see lectures on co-transporters and ATPase pumps). In particular the muscle fiber has: 1) Na+/K+ ATPase pump - to establish the electrochemical gradients of Na+ and K+ 2) Ca+2 ATPase pump - uses energy from ATP to remove 2 Ca+2 from the inside to the outside of the cell to ensure that internal Ca+2 concentrations remain low (10-7 mM internal). 3) Na+/Ca+2 cotransporter - to also remove Ca+2 from the inside of the cell and uses the energy from the cotransport of 3 Na+ molecules to export 1 Ca+2. 4) Muscle Ca+2 ATPase pump - a different pump from number 2 above. Found highly concentrated on the sarcoplasmic reticulum (SR) (constitutes 80% of the protein found in the SR membranes). The muscle Ca+2 ATPase pumps 2 Ca+2 into the SR to lower cytosolic Ca+2 and to concentrate Ca+2 into internal stores. Channels - muscle cells share many of the same ion channels as neurons. 1) leak channels - besides the leak K+ channel, skeletal muscle cells have a high concentration of Cl- leak channels so much so that the resting membrane potential is usually the same as the Nernst potential for Cl- (around -80 mV in these cells). The high permeability to Cl- helps repolarize the membrane after an action potential. 2) voltage-gated Na+ channels - there is a skeletal muscle voltage-gated Na+ channel which properties very much like the neuronal voltage-gated Na+ channel we have already discussed at length. TTX - sensitive as we saw above. This channel is of course responsible for the production of the action potential. Once opened the influx of Na+ through the channel further depolarizes the membrane thus opening more Na+ channels and this regenerative cycle continues until the Na+ channels start to inactivate and the delayed K+ channel begins to open. 3) voltage-gated K+ channel - the delayed rectifier K+ channel which we have already seen in neurons with exactly the same properties. In other words has a high threshold and needs a strong depolarization to open and works to bring the membrane back to resting potentials. 4) voltage gated Ca+2 channels - high threshold Ca+2 channels very much like we saw before at the neuronal chemical synapse. Needs a strong depolarization to open and very slowly inactivates. These Ca+2 channels are sensitive to DHP (dihydropyridine) and are found concentrated in the T-tubules. (Figure from "Cell Physiology Source Book") The average post-synaptic potential in a muscle fiber is a depolarization of 40 mV. The post-synaptic depolarization is always much greater than threshold for an action potential (rest is -80 to -70 mV and threshold for an action potential is -60 to -50 mV on average). This is to ensure that an action potential will be generated in the muscle fiber when ever a signal arrives at the NMJ. Triads and Sarcoplasmic reticulum The link between depolarization and Ca+2 release or excitation-contraction coupling occurs at the junctions between the T-tubule and the sarcoplasmic reticulum. 80% of the T-tubules membrane is associated with the sarcoplasmic reticulum at triads. The voltage-gated Ca+2 channels are concentrated in the T-tubules in the triads. The Ca+2 release channel found in the sarcoplasmic reticulum membrane is associated with the voltage-gated Ca+ 2 channel at this point. This close association allows for the rapid signalling from action potential to Ca+2 release. Figure 18-31B. (From "Molecular Cell Biology") The process that underlies excitation-contraction coupling (E-C coupling). The overall effect of an action potential arriving at the muscle fiber is that the voltage-gated Na+ channels in the membrane distribute the depolarization to the T-tubules which in turn depolarizes to open the voltage-gated Ca+2 channels. This results in a release of Ca+2 through the Ca+2 release channels from the sarcoplasmic reticulum. After the action potential is passed and the voltage-gated Ca+2 channels close, the Ca+2 release channels close and Ca+2 is recycled back into the sarcoplasmic reticulum through the Ca+2 ATPases. (Figure from "Cell Physiology Source Book") The voltage-gated Ca+2 channel is either closely localized to or makes a physical connection to the Ca+2 release channels in the sarcoplasmic reticulum. The voltage-gated Ca+ 2 channel is also known as the DHP receptor and the Ca+ 2 release channel is also known as the ryanodine receptor. Both these chemicals bind to their respective channels. As is illustrated in the diagram above not all Ca+2 release channels are associated with voltage-gated Ca+2 channels and these non-associated channels are thought to be opened solely by Ca+2 influx into the cytosol from the voltage-gated Ca+2 channels. (Figure from "Cell Physiology Source Book") The Ca+2 release channel in the sarcoplasmic reticulum of most muscle cells (smooth, cardiac, skeletal) is a Ca+2 activated Ca+2 channel. In other words the Ca+2 that enters through the voltage-gated Ca+2 channel from the outside of the cell triggers the opening of the Ca+2 release channel resulting in an efflux of Ca+2 from the sarcoplasmic reticulum into the cytoplasm. What is interesting is that the Ca+2 release channel is stimulated to open at with low concentrations of Ca+2 ( up to 0.1 mM) in the cytosol but inhibited by high concentrations of Ca+2 in the cytosol (0.5 mM and higher for cardiac cells). This means that as Ca+2 is released from the sarcoplasmic reticulum it starts to inhibit the Ca+2 release channel. This is necessary otherwise the Ca+2 release would be continual. In vertebrate skeletal muscle the Ca+2 release channel is stimulated to open in two ways through two different Ca+2 release channels: Ca+2 gated (see above in case of non-associated release channels) and mechanically gated. In vertebrate skeletal muscle cells the skeletal voltage-gated Ca+2 channel is physically linked to the Ca+2 release channel. As the voltage-gated Ca+2 channel goes through a protein conformational change during depolarization i.e as the voltage-sensor moves in the membrane during the depolarization, this opens both the voltage-gated Ca+2 channel and somehow this physical change is transduced to the the Ca+2 release channel. (see the notes on the voltage-gated Na+ channel and action potentials in Lodish et al. "Molecular Cell Biology" chapter 21). Figure 20-40. Release of Ca+2 stores mediated by Ca+2 release channel (ryanodine receptors) in skeletal muscle. (From "Molecular Cell Biology") Experiments: The following are examples of experiments that were carried out to prove the above models of muscle cell function. These experiments are based on a simple assay system that tests muscle function (i.e. contraction) under different conditions. (Adapted from "Cell Physiology Source Book") To test the physiology of muscle fibers the muscle fiber is dissected out and attached usually via its tendon to a solid surface. At the other end a the force of contraction is measured. The muscle fiber can be stimulated to contract through a number of experimental means, either by stimulating the motorneuron that is synapsed with the fiber or by directly depolarizing the muscle fiber with an injection electrode (B). In either case the force of contraction can be measured. (Figure from "Cell Physiology Source Book") Figure 2 shows that the degree of contraction or force exerted by the muscle fiber is dependent on the concentration of Ca+2 inside the muscle cell. As the Ca+2 concentration increases the force of the contraction increases to a maximal level. (Figure from "Cell Physiology Source Book") Figure 4 highlights the timing between excitation-contraction coupling. The first A shows the speed of the excitation (action potential) - contraction coupling. Tension or contraction is generated within milliseconds of the generation of the action potential. In B shows the time scale of the Ca+2 peak in response to each action potential. The fiber was stimulated with multiple action potentials. The peak in Ca+2 release that occurs with each action potential was measured using a Ca+2 sensitive dye such as Fura-2 (as previously discussed in the case of the detection of Ca+2 in the bitter taste transduction pathway). (Figure from "Cell Physiology Source Book") Figure 8 details the experiments that show that (in the absence of external Ca+2) simply depolarizing the muscle cell membrane beyond Ca+2 channel threshold is enough to generate muscle contraction or force in a vertebrate skeletal muscle fiber. If this experiment was carried out in a cardiac muscle cell then there would be no force generated. Cardiac, smooth and invertebrate skeletal muscle cells depend on the influx of Ca+2 into the cell from the outside to trigger the opening of the Ca+2 release channel. The above experiment in Figure 8 is one of the experiments that lead to the idea that it in vertebrate skeletal muscle cells it is not only Ca+2 entering through the voltage-gated Ca+2 channel that opens the Ca+2 release channel but must be a physical contact between the opening of the voltage-gated Ca+2 channel and the release channel. The proof for this idea came from the experiment outlined below. To test the hypothesis that it is physical contact between the skeletal voltage-gated Ca+2 channel and the Ca+2 release channel the experimentors used molecular techniques to alter the coding sequence of the skeletal voltage-gated Ca+2 channel and thus made mutant channels. They hypothesized that the interaction site would be on the intracellular side of the voltage-gated Ca+2 channel. To test this i) they systematically removed regions of the channel that are thought to be on the intracellular side or ii) replaced each intracellular region with the equivalent region from the cardiac voltage-gated Ca+2 channel (called a chimera protein) Each mutant channel was then expressed in mouse muscle cells that are lacking a functional voltage-gated Ca+ channel (called dysgenic myotubes as they can not contract). The following positive controls were used to test the system: i) Normal or wild type voltage-gated Ca+2 channels from skeletal muscle were expressed in the dysgenic myotubes and were able to make the mytotubes functional i.e. now the myotubes contracted in response to depolarization independent of external Ca+2. ii) Normal or wild type voltage-gated Ca+2 channels from cardiac muscle were expressed in the dysgenic myotubes and could only make the mytobues contract when external Ca+2 was present. Using this type of approach the researchers confirmed that a specific region on the intracellular side of the skeletal voltage-gated Ca+2 channel is responsible for triggering the opening of the Ca+2 channel in a voltage-dependent but Ca+2 independent manner. As well they confirmed that the cardiac voltage-gated Ca+2 channel does not have this region. (Tanabe et al. (1990) Nature 346: 567-569.) Actin and myosin in muscle contraction (Figure from "Cell Physiology Source Book") Figure 18-26. Stucture of skeletal and smooth muscle. (From "Molecular Cell Biology") Skeletal muscle is made up of bundles of multinucleate muscle cells (myofibers). Each cell contains myofibrils that are composed of repeated units of actin and myosin called sacromeres. This repeated unit of actin and myosin gives the muscle cell a striped or striated appearance. Smooth muscle does not have the same arrangement having only one nucleus and containing loose bundles of actin and myosin. Figure 18-27. Structure of the sarcomere. (From "Molecular Cell Biology"). A micrograph (a) and cartoon (b) of a mouse striated muscle sacromere showing the position of the Z disks which anchor the thin filaments (actin based) at the plus end of the actin filaments and the thick filaments (myosin based). At higher power (c) in the striated muscle of an insect, the myosin heads can be seen linking the thick and thin filaments. The Z disk is complex of proteins that results in the darkly electron dense region. This complex is responsible for anchoring the actin filaments to ensure that the sacromere will shorten during contraction. The actin filaments are capped at both ends to ensure that the actin will not depolymerize. In smooth muscle which do not have Z disks the dense bodies anchor the loose bundles of thin filaments at one end and at the other the actin filaments are anchored in the cell membrane at attachment plaques (similar in many ways to dense bodies). Figure 18-02. Structure of F-actin. (From "Molecular Cell Biology") Electron micrograph of an actin filament and model of actin. Actin filaments (F-actin) are made up of individual G-actin molecules that polymerize into filaments in a process that depends on ATP. The filament is oriented with a - and a + end, with polymerization occuring faster at the + end. In places where the cytoskeleton does not change, such as in muscle sacromeres, the ends of the actin filaments are capped to ensure stability. (left) Figure 18-03. Binding of myosin S1 head domain to an actin filament. The myosin heads when bound to actin filaments spiral around the actin. Figure 18-20A. Structure of some myosin molecules. (From "Molecular Cell Biology"). There are many members of the myosin family of proteins. Myosin II is involved in muscle contraction and cytokinesis. The heavy chain of myosin contains the head domain which is the ATPase domain that binds both actin and ATP. The neck region behind the head domain binds to the light chains and the tail domain of two myosin II molecules forms a rodlike structure of coiled-coiled domains of the myosin II dimer (see Figure 18-21B below). The light chains associated with myosin II are Ca+2 binding proteins that help regulate the movement of myosin along the actin filament. Figure 18-21B. Myosin II coiled tail domains. (From "Molecular Cell Biology"). The rodlike tail domains of the myosin II dimers associate to form the sacromere thick filament with a bipolar arrangment of head domains at either end. Figure 18-22. The sliding-filament assay. (From "Molecular Cell Biology"). Myosin heads slide or walk along the actin filament. Myosin molecules anchored to a glass slide will move actin filaments in the presence of ATP. Figure 18-22 diagrams an experiment where myosin molecules attached to a glass coverslip. Actin filaments that are labelled with a fluorescent dye are then added. In the absence of ATP there is no movement. In the presence of ATP there is movement. Figure B shows the movement of the labelled actin filaments at 30 second intervals. Myosin II from skeletal muscle has the fastest rates of movement. The myosin head moves in discrete steps (5-10 nm in the case of Myosin II) along the actin filament. Figure 18-25. Coupling of ATP hydrolysis to movement of myosin along the actin filament. (From "Molecular Cell Biology"). A model of how myosin moves is diagrammed in the above figure. It is still not absolutely certain that the myosin head moves once per ATP hyrolyzed but this is the current model. Myosin is bound to actin in the absence of ATP and this is the "rigor" state i.e. gives rigidity to the muscle. ATP binds to the myosin causing the head domain to dissociate from actin. ATP is then hydrolyzed causing a conformational change in the mysoin head to move it to a new position and bind to actin. Pi is released causing the myosin head to change conformation again and it is this movement that moves the actin filament. Figure 18-29. Sliding filament model of contraction in striated muscle. (From "Molecular Cell Biology") In the presence of ATP and Ca+2 the myosin heads, extending from the thick filaments, pivot pulling the actin thin filaments towards the center. The thin filaments are anchored and thus the movement shortens the sarcomere length. What would happen if the actin filaments were not anchored? This model predicts that the force of the contraction is proportional to the shortening of the sacromere or the overlap between the thin and thick filaments. Figure 4 below shows this where as the force of the contraction increases the length of the sacromere decreases see B and C. (Figure from "Cell Physiology Source Book") How does Ca+2 control the interaction between myosin and actin? As we have seen above Ca+2 is the controlling element for muscle contraction. In the absence of Ca+2 myosin remains bound to actin but can not move. How does Ca+2 control this interaction. Again this process differs in different muscle types. Figure 18-32. (From "Molecular Cell Biology") In vertebrate skeletal muscle the actin filament is associated with a protein complex that is made up of tropomyosin and troponins C, I and T. Tropomyosin is a ropelike protein that is strung together in a head to tail chain along the actin filament. Tropomyosin contains actin binding sites that ensures a tight interaction. The troponin complex of C, T and I are associated with tropomyosin and controls the location of the tropomyosin on the actin filament. Ca+2 binds to the troponin C subunit and somehow this interaction moves the troponin/tropomyosin complex on the actin filament. This movement now frees up binding sites for myosin II and allows the myosin II to move along the actin filament. Therefore while there is an increased concentration of Ca+2 then myosin is free to slide along the actin filament. Once the Ca+2 is removed back into the sarcoplasmic reticulum then the troponon/tropomyosin complex moves back and blocks the further movement of myosin along the actin. The sacromere relaxes and the system is set to contract again. Figure 18-33A. Regulation of skeletal muscle contraction by Ca+2 binding to troponin. Summary figure of vertebrate skeletal muscle contraction (Figure from "Cell Physiology Source Book") Skeletal muscle metabolism Muscles of course require a large source of ATP to allow for contraction and for transport of Ca+2. The ATP requirements are normally met by glycolysis or respiration. Frequently used high activity muscles use oxidative respiration mainly to generate ATP. These muscles have extremely extensive mitochondria which extend through out the myofibrils. They are red coloured due to a large blood flow and myoglobin (which stores oxygen). During intense activity though the supply of oxygen can not keep up with the demand to fuel oxidative phosphorylation even with the reserves from myoglobin. At this point anaerobic respiration kicks in and glucose is converted to lactate. This is not very efficient and only generate 2 ATP per glucose. Skeletal muscles contain large glycogen stores to help fuel ATP production. As well skeletal muscles contain creatine phosphate that generates ATP through : creatine phosphate + ADP = creatine + ATP This system actually maintains ATP levels at a similar level in inactive versus active muscles (this is not the case during intense activity). The breakdown of glycogen stores in muscles can be stimulated by both Ca+2 and epinephrine.