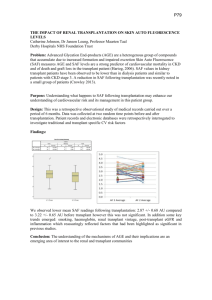
Transplant Manual
advertisement

Table of Contents I. Introduction II. Staff Directory and Frequently Used Numbers III. Organization of the Service IV. Personnel V. Dictation Instructions VI. Resident Responsibilities VII. Weekly Schedule VIII. Liver Transplant Guidelines IX. Kidney Transplant Guidelines X. Pancreas Transplant Guidelines XI. Dialysis Access XII. Common Complications XIII. Liver Transplantation Background XIV. Kidney Transplantation Background XV. Pancreas Transplantation Background XVI. Dialysis Access Background XVII. Immunosuppression Background XVIII. Infections XIX. Operative Notes XX. Appendicies The entire contents of this manual along with selected references can be found in the transplant shared disk drive. I. Introduction Facilitating outstanding patient care is the goal of this manual. Complicated transplant patients require highly educated caregivers, and so this book should be used as a study guide as much as a patient care guide. Residents will be expected to develop an in-depth knowledge of the information and concepts contained in this book and be able to apply their understanding to patient care. Comments and suggestions on how to improve the manual are greatly appreciated. II. Staff Directory and Frequently Used Numbers Page Office Home Cell 39288 39861 36104 39925 781-945-6467 617-632-9700 617-632-9700 617-632-9700 617-632-9700 617-632-9700 978-369-1776 781-235-1232 617-469-4414 617-327-1703 617-969-4682 617-797-1472 617-872-5259 617-276-6261 617-838-7828 90165 39126 91400 32605 33788 91651 617-632-1058 617-632-1089 617-632-9700 617-632-1064 617-632-1070 617-632-0831 617-241-8851 617-734-3604 781-449-3051 781-893-1950 617-277-9178 781-237-2307 857-891-6728 95761 781-317-8297 617-667-2371 617-632-9252 781-772-1024 617-731-2676 781-801-5428 617-784-2543 9010 95692 617-632-1070 617-632-1070 617-879-2612 781-402-0221 508-358-1640 617-795-2042 Transplant Surgeons Douglas W. Hanto, MD, PhD Scott R. Johnson, MD Seth J. Karp, MD Khalid Khwaja, MD Anthony P. Monaco, MD Transplant Hepatology Nezam Afdhal, MD Valerie Byrnes, MD Michael Curry, MD Sanjiv Chopra, MD (Thomas) Barry Kelleher, MD Simon Robson, MD, PhD 617-633-5036 GI Attending physicians Detlef Schuppan, MD, PhD Gary Trey, MD Hepatology Fellows-- call beeper 33002 Asim Khokhar, MD Seema Maroo, MD Transplant Nephrology Attending Physicians Martha Pavlakis, MD 38351 617-632-9700 Didier Mandelbrot, MD 33943 617-632-9700 617-283-7598 617-833-5821 Transplant Nephrology Fellow: Ogo Egbuna 32345 617-639-5572 Nephrology Attending Physicians: Robert Cohen, MD Neera Dahl, MD, PhD John D’Elia, MD Antoine Kaldany, MD Ananth Karumanchi, MD Stewart Lecker, MD Bijan Roshan, MD (Joslin) Theodore Steinman, MD Terry B. Strom, MD 34822 36606 82935 92920 33403 35350 82941 31997 617-667-2147 617-667-2147 617-732-2477 617-632-9888 617-667-1018 617-667-2147 617-732-2477 617-667-5278 617-632-0150 617-232-5989 617-576-7612 781-259-0472 617-969-0910 617-734-7530 617-277-6012 617-719-8501 867-636-8032 617-519-6386 Henry Yager, MD Mark Williams, MD Melanie Hoenig 81931 82923 617-243-5700 617-732-2477 617-244-6940 617-267-5652 617-497-9282 978-452-3402 781-405-9637 617-875-3493 Nephrology Fellows Aylit Schultz Farhad Chimeh GaborBodonyi-Kovacs Joshua Tarkan 37447 32344 32346 32347 617-277-3773 781-477-9992 617-412-7551 617-859-8516 33948 617-738-4679 33950 781-391-6086 39756 39756 39756 39756 39756 617-632-9700 617-632-9700 617-632-9700 617-632-9700 617-632-9700 508-423-7586 781-740-8050 781-812-2121 781-545-4528 978-685-3654 39756 617-632-9700 617-323-1726 92072 617-632-9814 781-767-3103 35909 617-632-8957 617-935-2740 Farr 9 Farr 5 Farr 3 CC-7 Stoneman 5 2-8738 2-7551 2-7171 4-3200 7-3355 East 7-4944 East 7-5227 Transplant PA Lori Romeo, PA-C Transplant NP Sandra Crawford-Zanger, NP Transplant Coordinators Erin Cavanaugh, RN Elizabeth (Betsy) Gray-Chrzan, RN Tina Healey, RN Kimberly Sullivan, RN Linda M. Walsh, RN 978-273-4696 Transplant and Dialysis Access Coordinator Louise Riemer, RN Transplant Listing Laura Aguiar Transplant Pharmacist Christin Rogers, PharmD Transplant staff pharmacist 92432 General Numbers Admitting Blood Bank Cath Lab CODE PAGE Dialysis Unit (Farr 7) Echo Lab ED Floors— Farr 10 Farr 7 Farr 4 Farr 2 CC-6 12 Reisman GI Units—West HLA lab ICU’s—Finard 4 (East) CSRU (5th Floor) NSICU (6th Floor) SICU-A SICU-B TSICU (6th Floor) CCU MICU A MICU B Information Systems Laboratory—West 4-2210 4-3300 2-7448 2-1212 2-8767 2-8955 4-2400 2-8731 2-8710 2-7448 2-7272 4-3150 7-3877 4-8434 732-5872 7-3124 4-2900 4-3250 4-3250 4-2930 4-3130 4-2930 4-3270 4-3180 4-8080 4-3230 617-935-2740 Medical Records New England Organ Bank OR—West Main Desk Pre-op Holding PACU OR—East Main Desk Feldberg Pre-Op Shapiro Pre-Op Phase 1 Recovery Phase 2 Recovery Pharmacy Radiology Main Angio CT Body RR ED—Main RR File Room MRI Neuro Reading Room RTAS (Dictations) Ultrasound RR Vascular Lab Rapa levels at Children’s Surgical Education Transplant Center 7-3703 800-446-NEOB 4-3000 4-3100 4-2800 7-2411 7-5663 7-0200 7-0300 7-4790 4-3283 West 4-2500 4-2552 4-2534 4-2300 4-2305/6 4-2538 4-2090 7-1100 7-7834 4-2628 4-2630 617-355-6351 2-9236 2-9700 East 7-2515 7-9515 7-2745 Boston Hospitals BIDMC BID-Needham (Glover) Boston Med Center (BUMC) Brigham & Women’s Cambridge City Children’s Terry McCarthy Dana Farber Joslin Mass Eye & Ear Massachusetts General Mount Auburn Debbie Lowe New England Baptist New England Medical Somerville 617-667-7000 781-453-3000 617-638-8000 617-732-5500 617-665-1000 617-355-6000 617-355-2469 617-632-3352 617-732-2400 617-523-7900 617-726-2000 617-492-3500 617-499-5150 617-754-5800 617-636-5000 617-591-4500 III. Organization of the Service Patients are cared for jointly by the transplant service, nephrology, and hepatology. Patients who have had a liver, kidney, or pancreas transplant are usually admitted to the transplant service with either hepatology or nephrology following as a consult. Patients with purely medical problems may be admitted to the nephrology or hepatology service. In this case, the transplant service should round daily on these patients as a consult. The close working relationship between the services means that these patients should be jointly managed and therefore recommendations made by the nephrology or hepatology services should generally be followed. An ICU consult should be obtained for all transplant patients in the ICU. The ICU staff and residents provide tremendous help in managing our patients. As much as possible decisions are made jointly between the services. When disagreements arise as they inevitably will with complicated patients, they should be resolved at the attending level. Surgical attending staff call staff is call is divided into 1) liver and hepatobiliary 2) kidney and pancreas, and 3) donor and access. Attending physicians generally rotate every week. All issues on a particular service should go through the fellow or chief resident to the attending on that service. For example, with few exceptions, all issues on liver inpatients, admissions and consults are handled by the attending on liver call for the week. Outpatient phone calls and issues are generally not the responsibility of the residents and should be referred to the transplant coordinator on call or the attending covering that service Emergencies are frequent on the service, and staff will frequently cover cases for each other. It is imperative that patients not be brought into an operating room unless the staff member that is actually going to do the case is aware the case is going to begin. Transplant inpatients have absolute priority for beds on Farr 10. Whenever possible, patients should be moved here, even if it means having to bump another patient to another floor. IV. Personel The transplant service is a multidisciplinary group that includes a large number of people with tremendous experience and diverse expertise. Farr 10 Staff We are fortunate to have an award winning, experienced nursing team caring for our patients. Monthly breakfasts to facilitate communication among the various staff members should be attended whenever possible. Nurses should be invited to attend staff rounds. Transplant Nurse Practitioner Nurse practitioners have a master’s degree in nursing and are certified by the American Nurses Credentialing Center. They assess, plan and coordinate pre- and post-transplant care. The NPs allow for continuity of care and permit the residents to take a greater operative role on the service. The NP provides direct patient care under the supervision of the surgeons and physicians and works closely with residents, fellows and the nursing staff. NP responsibilities include: Physical examination Documenting initial history and physical findings with treatment recommendations Admission orders as needed Evaluating patients for discharge and writing discharge orders which may include dictation of comprehensive discharge summaries Defining discharge regimen with the multi-disciplinary team Recommending follow-up consult referrals Direct referral to dietician, PT, OT, social services, pain/palliative care teams, behavioral medicine, speech and swallowing therapists Formulation of patient care plans Ordering diagnostic tests Educating patients, families and caregivers Liaison between the Transplant Unit and visiting nurses, clinic staff and consult services Rounding with the transplant team Informed consent: surgery, routine procedures, blood products Procedures: Placement and removal of NG tubes, removal of drains, minor suturing Interpretation of EKGs and chest X-rays Prescriptions of Schedule II-VI drugs Physician Assistant PA’s take courses on a medical student model which consists of a premed program of one year of didactic coursework, and then more than a year of clinical rotations. This results in a baccalaureate or master’s degree in health sciences as a physician assistant. Certification as a PAC comes after passing a recertification exam. Similar to the nurse practitioner, the purpose of the PA on the transplant service is to contribute to continuity of care and allow the residents to devote their time more to the operating room. Duties of the physician assistant include: Physical examination and assessments Recording and presenting pertinent physical data to the physician Performing and/or assisting in laboratory and screening procedures delegated by the physician Performing or assisting in approved therapeutic procedures delegated by the physician Arranging hospital admissions by completing forms and charts pertinent to the pa medical record Transplant Coordinators Coordinators play a critical role in outpatient management. They generally follow the patients for long periods of time and know them very well. Coordinators are the first line of contact for outpatients with problems and so the coordinators play a critical role in evaluation and management. Other duties include follow-up of outpatient laboratory values, scheduling tests, communicating medication changes, and arranging for services. It is imperative that coordinators be apprised of discharge medications and in-house issues so they can be appropriately considered after the patient leaves the hospital. V. Dictation Instructions Operative notes will be dictated by the attending involved in the case unless specifically discussed. Discharge summaries should be dictated the day of discharge and are the responsibility of the residents on the service. Please follow the instructions on the printed cards and include a detailed past medical history and a brief summary of the hospital course including review of relevant studies including ERCP’s, biopsies, and CT scans. VI. Resident Responsibilities Most attending physicians have four ways to be reached including home and cell numbers. The importance of communication cannot be overemphasized when taking care of complicated patients. Attending physician should be apprised immediately of all admissions, discharges, consults, significant changes in patient status, and kept up to date when issues arise. Most of us have long-term relationships with our patients and families, and our knowledge of accurate, timely information facilitates this relationship. Residents should write daily notes on patients including primary diagnosis and ongoing issues. Typically this is done by the night float. Additional notes should be added when there is a significant event or change in a patients condition. Consult patients should also be seen daily and a note written. Schedule changes should be considered the rule on the service. For this reason, no patient should be brought into the operating room unless the resident has communicated with the attending and knows the attending is in house and agrees that the case should proceed. Residents will be expected to be knowledgeable about the patient and the operation to be performed. Under no circumstances should a resident show up unprepared and expect to operate. The resident should meet the patient, verify the consent is signed, the appropriate site is marked, and there are no anesthesia delays. Each resident should be familiar with all the patients on the service. On joining the service, each resident will be provided with a password to the Organ Transplant Tracking Record (OTTR) to facilitate communication of the outpatient events. Residents are encouraged to consult OTTR as necessary. VII. Weekly Schedule Monday Tuesday Wednesday 7 am Morbidity and Mortality Conference 8 am Grand Rounds 9 am Liver Clinic LMOB 7 10 am Liver Clinic LMOB 7 11 am Liver Clinic LMOB 7 12 pm Liver Meeting LMOB 7 Tumor Conference Shapiro 4 1 pm Transplant Walk Rounds 1:30- Liver Clinic LMOB 7 2 pm Liver Clinic LMOB 7 3 pm Liver Clinic LMOB 7 4 pm Transplant Lecture 4:30-5:30 Kidney Meeting LMOB 7 5 pm VIII. Liver Transplantation Guidelines Pre-transplant admission Thursday Friday Combined HB/Transplant Teaching Conference Any concerns should be communicated IMMEDIATELY to the attending to decide if the case should proceed. Work-up should proceed EXPEDITIUOUSLY in recognition that the organ is on ice and time is of the essence. CXR, labs, EKG and H and P should be done IMMEDIATELY on patient arrival to floor Consult: Joslin for all diabetic patients or if patient becomes diabetic Nephrology if ESRD Hepatology Anaesthesia History: Complete including recent infections, hospitalizations, allergies, medications Physical: Complete including signs of infection Labs: CBC w/diff, Chem 7, PT, PTT, INR, fibrinogen, LFTs, Ca, PO4, Mg, U/A Studies: CXR, EKG Blood set-up: Type& cross 10 prbc, 10 ffp, 10 platelets, 10 cryoprecipitate Weight Consent Meds: Mycophenolate Mofetil 1g IV on call to OR Fluconazole 400mg orally on call to the OR Methylprednisolone 500 mg IV on call to OR Ampicillin/Sulbactam 3g IV given prior to incision (If PCN allergy then Vancomycin 1g IV and Levofloxacin 500mg). Redose every 8 hours in OR. Heparin 5000U SC on call to OR Review and check consent on any studies patient entered in Check HBsAb, HBV DNA for patients with hepatitis B HCV quantitative evaluation blood in patients with hepatitis C In operating room Surgical shave with clippers Signed ABO compatibility sheet Check with organ bank correct liver was received Foley catheter, CVP, Swan-Ganz, NG tube, dialysis line as needed Compression boots prior to intubation Cell saver unless patient has tumor Confirm administration of antibiotics and immunosuppressives Hepatitis B Immunoglobulin (HBIG) 10,000 units IV during anhepatic phase in patients with a prior history of Hepatitis B or those who are receiving a HbcAb liver (See HBIG protocol) Post-operative Orders Admit to SICU s/p Liver Transplant Condition Vitals per ICU routine, daily weights Allergies Foley to gravity, Swan-Ganz to continuous monitoring, NGT to low wall suction, JP x 2 to bulb suction, biliary draininage open to gravity NPO IV: Discuss with attending or fellow Meds: Tacrolimus 0.05 mg/kg bid (consider lower dose if renal failure) Mycophenolate Mofetil 1 gm IV Q 12 hours Methylprednisolone taper- POD1-200mg IV, POD2-150mg IV, POD3-100mg IV, POD4-70mg IV, POD5- 35mg IV, POD6-20 20 mg PO daily Gancyclovir 5mg/kg/IV q 12 hours (adjust for renal failure) Fluconazole 400mg ng q day Sulfamethoxazole/trimethoprim suspension 10 mL’s pNGT q day. (If sulfa allergy, pentamadine 300 mg via inhalation monthly) Pantoprazole 40 mg IV q day (when taking PO) Heparin 5000u sc tid Dolasetron 12.5 mg iv q8 prn Morphine 2-5mg IV q 1-2 h Ampicillin/Sulbactam 3g q 8 IV x 24 hours. (If penicillin allergic Vancomycin 1000mg q 12 IV and levofloxacin 500mg IV q 24 Bed rest, Turn q 6 hours Vent management as appropriate Compression boots CBC,chem. 7, PT, PTT, INR, fibrinogen, LFTs, amylase, albumin, Ca, Po, Mg q 8 hr CXR POE order set completed Post-op Management Labs: CBC, chem 7, LFTs, PT, PTT, INR, Fibrinogen (q 12h) until on floor, then QD Prograf, C2, or rapa level QD, ABG BID until extubated CXR QD until extubated Hepatic Doppler on POD 1 Accucheck qid as needed Daily weights Meds: Tacrolimus 0.05 mg/kg bid (consider lower dose if renal failure). Target level 10-12 first 6 months, 8-10 6 months to one year, then 6-8 Mycophenolate Mofetil 1 gm IV Q 12 hours, change to po when tolerating Methylprednisolone taper- POD1-200mg IV, POD2-150mg IV, POD3-100mg IV, POD4-70mg IV, POD5- 35mg IV, POD 6-20 20 mg PO daily then decrease by 2.5 mg every 10 days unless prior rejection Gancyclovir 5mg/kg/IV q 12 hours (adjust for renal failure), then Valcyte Fluconazole 400mg q day Sulfamethoxazole/trimethoprim suspension 10 mL’s pNGT q day. (If sulfa allergy, pentamadine 300 mg via inhalation monthly), change to po when tolerating Pantoprazole 40 mg po q day (when taking PO) Heparin 5000u sc tid Morphine 2-5mg IV q 1-2 h PRN, then oxycodone 5-10 mg po q4-6 hr prn Ampicillin/Sulbactam 3g q 8 IV x 24 hours. (If penicillin allergic Vancomycin 1000mg q 12 IV and levofloxacin 500mg IV q 24 Dolasetron 12.5 mg IV q8 prn Propofol if intubated and needed. Titrate to effect Pamidronate 0.5mg/Kg prior to discharge from transplant stay (one time, round to 30 or 60 mg) JP: bulb suction, strip and record q one hour while in ICU, then q4 hours. Consider removal when volume less than 100/day if non-bloody and non-bilious. T-tube: gravity and record q 1hr until transfer to floor, then record q shift. Study on POD 5-7. Foley: gravity, d/c when patient ambulating and stable A-line: d/c on transfer to floor NGT: Flush q shift with 10 cc NS. Consider removal if abdomen soft and passing flatus IVF: Consider D5 ½ NS @100cc/hr depending on fluid status, discuss with attending Nutrition: Start TF as soon as possible, advance diet as tolerated. Nutrition consult, calorie counts x 3 days VRE and wound assessment by house staff Extubate, wean per protocol O2 to keep sat. >92%, D/C if sats above 92 on RA for 15 minutes Incentive spirometer q1 hour Cough pillow while awake Compression Boots Transfer to Farr 10 when extubated and stable Social Work assessment, Psych. consult if history of psych disorder OT Consult to evaluate functional performance Consider furosemide Restart home meds as appropriate Remove dressings on POD 2 Independent walking program, exercise prescription as established by PT Hepatitis B Therapy HBV non-replicator (HBsAg+, HBeAg-, HBeAb+, HBV DNA+ HBV replicator (HBsAg+, HBeAg+, HBeAb-, HBV DNA+ Recipients of HBcAb+ donors to non-immune recipients 10,000 U IV while anhepatic, then 5000 U IV x 5 days to ensure anti-HBs >500 u/L and HBsAg-. Then 5 mL’s IM on days 7,14,21,28 10,000 U IV while anhepatic, then 5000 U IV x 5 days to ensure anti-HBs >500 u/L and HBsAg-. Then 5 mL’s IM on days 7,14,21,28 10,000 U IV while anhepatic, then 5000 U IV x 5 days to ensure anti-HBs >300 u/L and HBsAg-. Then 5 mL’s IM on days 7,14,21,28 Redose with 5 mL(>1560 U) when anti-HBs < 500 u/L for the first year and when antiHBs < 150 thereafter Redose with 5 mL(>1560 U) when anti-HBs < 500 u/L for the first year and when anti-HBs < 150 thereafter Lamivudine 100 mg/day beginning day of surgery 100 mg/day beginning prior to transplant. If resistant, adefovir 10 mg/day 100 mg/day beginning day of surgery Labs Quant HBsAb and HBsAg QD (in lab by 8 am) x first 5 days then before HBIG dose on days 7, 14, 21,28 and q 2 weeks for 3 months and monthly thereafter Quant HBsAb and HBsAg QD (in lab by 8 am) x first 5 days then on days 4, 14, 28 and q 2 weeks x 1 year. Quant HBsAb and HBsAg QD (in lab by 8 am) HBIG 5000 U IV if INR > 1.4 and IM administration of 5 mL’s is unsafe HBIG 5000 U IV if INR > 1.4 and IM administration of 5 mL’s is unsafe HBIg Other Redose with 5 mL(>1560 U) when anti-HBs < 500 u/L for the first 3 months Recheck HBV at 6 weeks. Obtain HBV results on donor from organ bank HBIG 5000 U IV if INR > 1.4 and IM administration of 5 mL’s is unsafe Lamivudine and Adefovir are renally excreted and need to be dosed according to renal function. At any stage, if there is development of HBV resistance as defined by re-emergence of HBV in spite of compliance and adequate anti-HBs titers, the patient should be treated with Adefovir 10mg po day (dosed as per renal function) Fulminant Liver Failure-ICP Monitoring Criteria: Onset of hepatic encephalopathy within 12 weeks of jaundice Grade III-IV hepatic encephalopathy or clinical signs of raised ICP in a ventilated patient. Candidates for OLT or have a good prognosis by conventional criteria (King’s College, see fulminant liver failure in background section) Evaluation: Head CT prior to catheter placement to r/o intracranial bleed or other pathology PT, PTT, INR, Platelet count, fibrinogen Pre-procedure: Correct platelets to >100,000/uL Correct fibrinogen to >150 mg/dL 2 units of FFP then a dose of rFVIIa (40mcg/kg) STAT PT/ INR drawn with a goal of an INR<=1.4 Neurosurgery will place intraparenchymal catheter within 20 minutes of rFVIIa infusion Post-procedure PT, INR, PTT, platelets q 3 hours after placement. FFP or rFVIIa (20 mcg/kg) as needed to keep INR<=1.4 for the first 48 hours then INR< 2.0. CBC and fibrinogen every 24 hours Transfuse to platelet count >75,000/μL. If uremic, cryo and DDAVP may be administered Transfuse to fibrinogen > 100 mg/dL Maintain ICP below 20mmHg. Maintain CPP (MAP-ICP) above 70mmHg Mannitol 1-2mg/Kg for raised ICP. May repeat as long as serum osmolality < 320 mosm/L Consider CVVHD or ultrafiltration to remove volume if renal failure Catheter removal: Patient has recovered spontaneously, after OLT, or prognosis has worsened INR <=1.4 Platelet >100,000/uL Fibrinogen >150 mg/dL Tube review We routinely use the following tubes: Jackson-Pratt drain: These tubes are placed in the operating room, usually one beneath the biliary anastamosis and another under the suprahepatic caval anastamosis. They should remain on bulb suction and be stripped every shift. T-tube: This is a biliary tube placed into the common bile duct with a limb retrograde into the common hepatic duct and another toward the duodenum. This tube is placed if there is any concern for the biliary anastamosis. The tube can be used as a biliary access for studies. Roux tube: When a Roux-en-Y biliary anastamosis is performed this tube is generally placed. It travels from the bile duct through the anastamosis into the jejunum. It exists through a hole in the jejunum and continues through the abdominal wall and out the skin. Mild/Moderate Rejection Steroid Bolus x 3 Response- ↓ in LFT’s by 50% Yes Yes Rebiopsy 7-10 days after initiaWas patient on tion of therapy steroids No No 2 more days of steroid pulses Rebiopsy 7 days after initiation of Rejection RePersistent rejectherapy solved tion OKT3 Continue preAssess prerejection dose rejection immuSub therapeutic Appropriate C2 nosuppression C2 levlevels/Tacrolimus Maximize current Restart on Predels/Tacrolimus trough and MMF immunosuppresnisone 20 mg troughs or MMF dose sion daily dose 1 Rebiopsy at the end of OKT3 2Assess need for treatment 2 tacrolimus conversion if on cyclosporine Rejection therapy dosing Steroid bolus- 500 mg IV daily x 3 days, administer x 5 days if no response after 3 days OKT3 2.5-5.0 mg IV daily x 7days 1) Prior to initiation of OKT3 presence of anti-murine antibodies should be evaluated. Anti-murine antibodies should be checked weekly while on OKT3 or if inadequate response is observed. CD3 counts should be monitored daily to ensure a CD3 count (absolute #) < 25. If there is a persistent rise in CD3 count or if Cs3 count is greater than 50 increase OKT3 dose to 5 mg daily. 2) Patients should be converted from cyclosporine to tacrolimus if they develop a steroid resistant rejection while being maintained on therapeutic Neoral levels or if a second rejection occurs within a 4 week period of time. IX. Kidney Transplantation Guidelines Pre-transplant admission Any concerns should be communicated IMMEDIATELY to the attending to decide of the case should proceed. Work-up should proceed EXPEDITIUOUSLY in recognition that the organ is on ice and time is of the essence. CXR, labs, EKG and H and P should be done IMMEDIATELY on patient arrival to floor Consult: Renal Joslin if diabetic Anesthesia History: Complete including last dialysis, recent infections, how much urine made, recent chest pain, shortness of breath, claudication, prior abdominal operations Physical: Complete including femoral and pedal pulses, signs of infection Labs: CBC w/diff, Chem 7, PT, PTT, INR, fibrinogen, LFTs, Ca, PO4, Mg, Alb, U/A, culture Studies: CXR, EKG Blood set-up: T&C 2 units Check CMV serology: if IgG Positive, do nothing. If no results or IgG negative more than 3 months ago, send CMV titers Surgery consent Consider for research protocols NPO Meds: Unasyn 3.0g IV (30-60mins. Prior to incision) OR Ancef on call to OR Mycophenolate Mofetil 1 gm IV on call to OR Methylprednisolone 500 mg IV on call to OR In Operating Room Place standard or 3-way foley with irrigation set-up (check with attending), fill bladder by gravity. Anti-thymocyte globulin 1.25 mg/Kg IV on call to OR (round to nearest 25 mg, max 125 mg). To be given prior to reperfusion over 6 hours after steroids Heparin SC Pneumo boots Mannitol/lasix per attending prior to reperfusion Post-op Orders Admit to Farr 10 Vitals per routine Consider EKG CXR Labs: CBC, chem 7, Ca, Mg, PO4, alb, CyA, FK, rapa QD, accucheck Q6 hours if diabetic Meds: Anti-thymocyte globulin 1.25mg/kg (max 125), given over 6 hours, for 3-5 days depending on renal function. Givehalf dose if platelets < 80,000 OR wbc 2000-3000. Hold if platelets < 50,000 OR wbc < 2000. Tacrolimus 0.1 mg/kg bid, target through level 10-15 initially. Start POD 1 if good renal function, POD 3 or 4 if renal function poor. Consider cyclosporine (instead of tacrolimus) in hepatitis C positive, non-diabetic African-American or Hispanic recipients to decrease risk of diabetes Mycophenolate Mofetil 1 gm po bid Steroid rapid wean protocol: Methylprednisolone 100 mg IV POD 1, 50 mg IV POD 2, 50 mg POD 3, then prednisone 25 mg po on POD 4 and 5, then stop Valganciclovir 900 mg po q day (dose adjust for renal failure) Nystatin 5cc Swish and Swallow QID Sulfamethoxazole/Trimethoprim SS One tab po q day (If sulfa allergy, pentamadine 300 mg via inhalation monthly) Ampicillin/Sulbactam IV q6 x 24 hours Heparin 5000u sq tid until ambulatory Dolasetron 12.5 mg iv q8 prn Pantoprazole 40mg po q day Restart home meds as appropriate--Do not restart statins, ACE inhibitors, ARBs Immunosuppressive meds(see below) IVF: D5 1/2NS @50 cc + 1/2 NS @cc/cc urine output JP to bulb suction PCA (morphine, demerol, or dilaudid) Sips if tolerated Foley catheter O2 by face mask Pneumatic booots Post-op Management Labs: CBC, chem 7, Ca, Mg, PO4, CyA, FK, rapa QD Accucheck qid as needed Daily weights Meds: Anti-thymocyte globulin 1.25mg/kg (max 125), given over 6 hours, for 3-5 days depending on renal function. Givehalf dose if platelets < 80,000 OR wbc 2000-3000. Hold if platelets < 50,000 OR wbc < 2000. Tacrolimus 0.1 mg/kg bid, target through level 10-15 initially. Start POD 1 if good renal function, POD 3 or 4 if renal function poor. Consider cyclosporine (instead of tacrolimus) in hepatitis C positive, non-diabetic African-American or Hispanic recipients to decrease risk of diabetes Mycophenolate Mofetil 1 gm po bid Steroid rapid wean protocol: Methylprednisolone 100 mg IV POD 1, 50 mg IV POD 2, 50 mg POD 3, then prednisone 25 mg po on POD 4 and 5, then stop Valganciclovir 900 mg po q day (dose adjust for renal failure) Nystatin 5cc Swish and Swallow QID Sulfamethoxazole/Trimethoprim SS One tab po q day (If sulfa allergy, pentamadine 300 mg via inhalation monthly) Ampicillin/Sulbactam IV q6 x 24 hours Heparin 5000u sq tid until ambulatory Dolasetron 12.5 mg iv q8 prn Pantoprazole 40mg po q day Restart home meds as appropriate--Do not restart statins, ACE inhibitors, ARBs PCA, d/c when reliably toerating po’s IVF: D5 1/2NS @50 cc + 1/2 NS @cc/cc urine output initially, then decrease to 1/2 cc/cc urine output on POD 1 or 2, then maintenance fluids. Adjust for renal failure, be wary of slowing fluids if patient not tolerating po’s JP: bulb suction, strip and record q shift. Consider removal for output <60 cc/day Foley: d/c POD 3 or 4 for standard anastamosis with no hematuira or intraoperative issues. POD 4 for single stitch, if no hematuria or other issues. Nutrition: As tolerated unless abdominal complaints O2 by mask or cannula to keep O2 sat. >92%, D/C if sats above 92 on RA for 15 minutes Incentive spirometer q1 hour Cough pillow while awake Compression Boots Remove dressings on POD 2 Independent walking program re: exercise prescription as established by PT Transplant medication teaching by transplant coordinators with patient/family Diabetes management assessment Clinic appoinment one week after discharge Remove Triple Lumen catheter as soon as possible Do not use Kayexelate in a recent post-op patient X. Pancreas Transplantation Guidelines Pre transplant admission Any concerns should be communicated IMMEDIATELY to the attending to decide of the case should proceed. Work-up should proceed EXPEDITIUOUSLY in recognition that the organ is on ice and time is of the essence. CXR, labs, EKG and H and P should be done IMMEDIATELY on patient arrival to floor. Consult: Renal Joslin Anaestheia History: Complete including recent infections, chest pain, shortness of breath, claudication, prior abdominal operations Physical: Complete including femoral and pedal pulses, signs of infection Labs: CBC w/diff, Chem 7, PT, PTT, INR, fibrinogen, LFTs, lipase, Ca, PO4, Mg, Alb, U/A, culture Studies: CXR, EKG Blood set-up: T&C 2 units Check CMV serology: if IgG Positive, do nothing. If no results OR IgG negative more than 3 months ago, send CMV titers Surgery consent Consider for research protocols NPO Meds: Unasyn 3.0g IV (30-60mins. prior to incision) Fluconazole 400 mgIV (30-60 mins prior to incision) In Operating Room Hct/Chem7/Coags Accucheck Anti-thymocyte globulin 1.25 mg/Kg IV on call to OR (round to nearest 25 mg, Max dose 125 mg). Given Over 6 hours after steroids and prior to reperfusion Mycophenolate Mofetil 1 gm IV on call to OR Methylprednisolone 500 mg IV on call to OR Unasyn 3.0g IV--redose in 6 hrs if necessary. Regular Insulin IV infusion to deep BS 100-150 Mannitol 12.5-25g IV/Lasix 20-40 mg prior to reperfusion. Pneumatic boots (placed prior to intubation). Overnight in PACU Post OP: CBC/Chem 7/Coags/Amylase/ Lipase/CXR/ EKG, Accucheck Immunosuppressive meds per protocol (IV or per NG) Unasyn Ampicillin/Sulbactam 3.0g IV q6h0 ASA 81 mg per Ng Heparin infusion @200 units/hr, start 6-8 hours post-op (AFTER transplant attending approval) IVF: NS @75-125 cc/0 Regular Insulin IV infusion to keep BS 100-150 mg/dl-Call attending prior to beginning NG to LWS/JP to bulb suction PCA (morphine, demerol, or dilaudid) NPO Foley catheter O2 by face mask Pneumatic boots Post-operative Management Labs: CBC/Chem7/coags/Amylase, lipase/Ical/mg/phos QD Joslin consult Accucheck q1 hour on POD 1, then q2 hours on POD 2, then Q4 hours, then QID on POD 4 Pancreas US if indicated Meds: Anti-thymocyte globulin 1.25mg/kg (max 125) for 3-5 days depending on renal function. Half dose if platelets < 80,000 or wbc 2000-3000. Hold if platelets < 50,000 OR wbc < 2000. Tacrolimus 0.1 mg/kg bid, target through level 10-15 initially. Start POD 1 if good renal function, POD 3 or 4 if renal function poor. Mycophenolate Mofetil 1 gm po bid Steroid rapid wean protocol: Methylprednisolone 100 mg IV POD 1, 50 mg IV POD 2, 50 mg POD 3, then prednisone 25 mg po on POD 4 and 5, then stop Valganciclovir 900 mg po q day (dose adjust for renal failure) Sulfamethoxazole/Trimethoprim 10 mL’s PNG q day(If sulfa allergy, pentamadine 300 mg via inhalation monthly) Ampicillin/Sulbactam IV q6 x 3 days Heparin 5000u sq tid until ambulatory Nystatin 5cc Swish and Swallow QID Dolasetron 12.5 mg IV q8 prn Pantoprazole 40mg IV q day ASA 81 mg PO/NG QD Heparin increase to 400units/hour x 5 days (AFTER transplant attending/Fellow approval) ReguIar insulin IV infusion to keep BS 100-150 mg/dl Unasyn 3.0g IV q 6 hours x 3 days Valcyte 900 mg PO/NG QD (dose adjusted for renal function) Bactrim SS One tab per PO/NG QD IVF: DS ½ NS @75-125cc/0 NG to LWS (may use for meds). D/C on POD 5 if no abdominal complaints and passing flatus JP to bulb suction Transplant medication teaching by transplant coordinators with patient/family PCA Diet: Consider clear liquids on POD 5 if NGT out and no abdominal complaints Out of bed to chair as tolerated, out of bed for 3 hours and ambulate on POD 2 Foley catheter--d/c POD 3 unless issues O 2 a to keep sats above 92%. D/C POD 3 if sat above 90% after 15m RA Incentive Spirometer q1 hour while awake Pneumatic boots D/C surgical dressing POD 2 Social work assessment PCA, then percocet when tolerating po’s TED stockings after pneumo boots d/c’d Clinic appointment one week after discharge XI. Dialysis Access Pre-operative Evaluation Detailed history and physical including recent infection, previous lines previous access, arm swelling, peripheral vascular disease, coronary disease, pulmonary disease, Allen’s test. Labs: CBC, SMA7, usually will need potassium just prior to surgery unless dialyzed that day Discussion of hemodialysis versus peritoneal dialysis Consider central venogram if multiple prior lines, arm swelling, or prominent veins on upper arm Postoperative Generally done as an outpatient procedure but certain patients will be admitted to the medical service to initiate dialysis Check radial pulse, hand temperature, and capillary refill in PACU prior to discharge HAND pain or parathesias are not normal and require call to attending, may represent steal Return to clinic in two weeks Patients should be counseled to expect some swelling Louise Riemer is the dialysis access coordinator. Whenever possible keep her informed of patient’s progress and follow-up instructions. XII. Common Complications—Work-up and Treatment Many patients require readmission after transplant for a variety of complications. Detailed history and physical exam is the critical first step. Included in this is a thorough accounting of where the patient’s tubes are and what each is (supposed to be) doing. Liver Specific Elevated liver function tests Differential Diagnosis: Rejection, hepatic artery stenosis or thrombosis, portal venous stenosis or thrombosis, hepatic vein stenosis or occlusion, bile leak, biloma, biliary stricture, infection, dehydration, tube malfunction, recurrent disease including hepatitis C, heart failure, medications Work-up: Varies by signs and symptoms. After detailed history, physical and labs, consider ultrasound, tube study, angiogram/venogram, biopsy Treatment: Specific to cause Abdominal pain Differential Diagnosis: Biloma, anastamotic breakdown, cholangitis, hepatic outflow obstruction, hematoma, any other general surgical problem causing abdominal pain including obstruction, perforated viscus, ulcer, esophagitis, gastritis Work-up: CBC, LFTs, abdominal ultrasound, abdominal CT scan, consider endoscopy Treatment: Disease specific Elevated creatinine Differential Diagnosis: Usually not from intrinsic renal disease. Calcineurin toxicity, other meds, liver failure, infection, dehydration, diarrhea, nausea, vomiting, heart failure, cryoglobulins Work-up: History and physical, cultures, full laboratory evaluation Treatment: Treat underlying disease, consider lowering calcineurin inhibitors or converting to rapamycin Edema Differential Diagnosis: Volume overload, congestive heart failure, suprahepatic caval stenosis, renal insufficiency, deep venous thrombosis Work-up: Complete history and physical, full laboratory evaluation, consider ultrasound of lower extremities, especially if swelling is unilateral. Consider lasix, cardiac evaluation, venogram. Treatment: Specific to diagnosis Kidney Specific Elevated creatinine/Decreased urine output Differential Diagnosis: Acute rejection, chronic rejection, dehydration, medications especially high tacrolimus or cyclosporine levels, urinary tract infection, renal artery stenosis/thrombosis, renal vein stenosis/thrombosis, ureteral stricture, urine leak, lymphocloele, urinary retention, systemic infection, localized infection (e.g. BK virus), recurrence of original disease Work-up: Complete history and physical exam, examination of current medications, CBC with differential, metabolic panel, U/A, culture and sensitivity, transplant ultrasound Treatment: Specific to diagnosis. Specific points: The diagnosis will often be obvious after the initial work-up. It is important to determine volume status. If there is an intrinsic renal problem patients will tend to be volume overloaded and should not get fluid which can put them into congestive heart failure. Biopsy is the definitive mechanism to diagnose rejection and can also provide information regarding the underlying health of the kidney. Contrast studies are useful to define ureteral abnormalities. Nuclear medicine scan can help diagnose a leak but do not exclude a leak if normal. Pain over the Graft Differential diagnosis: Acute rejection, vascular compromise, infection, hematoma, lymphocoele, hydronephrosis Work-up: Complete history and physical exam, full laboratory evaluation, STAT transplant ultrasound Treatment: Specific to cause Hydronephrosis Differential Diagnosis: Ureteral-pelvic junction stenosis, ureteral stricture, ureteral-veiscular junction (anastamotic) stricture, stones, hematoma, urine leak. Mild hydronephrosis after kidney transplant is common and of no consequence if creatinine is normal Work-up: History and physical, ultrasound, consider contrast study or nuclear medicine scan Treatment: Consider stent, either retrograde or percutaneously for ureteral problems Pancreas Specific Abdominal Pain Differential Diagnosis: Pancreatic leak, anastomotic leak, peripancreatic infection/collection, pancreatitis (in bladder-drained grafts), graft thrombosis, small bowel obstruction Work-up: History and physical, hct, wbc, amylase and lipase, pancreatic ultrasound, CT of abd/pelvis with IV and oral contrast. Consider pancreatic biopsy (CT-guided) if acute rejection suspected Treatment: Specific to cause, consider percutaneous drainage of collection, NGT decompression, surgical exploration for anastomotic leak, thrombosis, or ongoing deterioration, foley catheter for graft pancreatitis in bladder-drained grafts, treatment for acute rejection if indicated Elevated Amylase/Lipase Differential Diagnosis: Acute rejection, pancreatic or anastamotic leak, peripancreatic infection or collection, pancreatitis (in bladder-drained grafts), graft thrombosis Work-up: History and physical, hct, wbc, amylase and lipase, urine amylase in bladder-drained grafts (will be low in acute rejection), pancreatic duplex, CT of Abd/pelvis with IV and oral contrast. Consider pancreatic biopsy (CT-guided) if acute rejection suspected Treatment: Percutaneous drainage of collection, treatment for acute rejection if indicated. Surgical exploration for anastomotic leak, thrombosis, or ongoing deterioration. Foley catheter for graft pancreatitis in bladder-drained grafts Hyperglycemia/sudden increase in insulin requirement Differential Diagnosis: Graft thrombosis (arterial or venous), acute rejection (uncommon in early postop period), prograf (FK 506) or steroid-related. Work-up: STAT pancreatic duplex, abd/pelvis CT pancreatic biopsy if duplex normal Treatment:Urgent surgical exploration, thrombectomy if possible, or graft excision. Treatment for acute rejection if indicated. Consider substituting cyclosporine or rapamycin for FK 506, lowering or eliminating steroids. Note: Fever, nausea and vomiting, albeit non-specific symptoms, may suggest a pancreatic or peripancreatic process (ie. graft leak, infection) and merit investigation. Gastroparesis is common in diabetics and may cause persistent postoperative nausea and vomiting; treatment is with NGT decompression, anti-emetics and prokinetic agents. General Fever Differential Diagnosis: Infection (bacterial, viral, fungal) medications, rejection, malignancy, poor pulmonary toilet Work-up: Complete history and physical exam, CBC with diff, U/A, blood and urine cultures, CXR, CMV PCR, HSV, VZV, stool studies. Treatment: Cause specific Leukopenia (WBC < 3000) Differential Diagnosis: CMV infection, other viral infection, medications, sepsis Work-up: CMV PCR, EBV, HSV, VZV, parvovirus, mononucleosis, medication review Treatment: Treat for CMV or other infection. Consider decreasing cellcept or valcyte. Consider Neupogen (G-CSF) 5mcg/ kg SQ until WBC > 5000 Cellcept Dose Reductions for Leukopenia WBC cells/µL Reduction in MMF dose > 2500 cells/µL None <2000-2500 cells/µL Cut dose in half <2000 cells/µL Hold <1000 cells/µL or ANC<1000 cells/µL Hold and Neupogen 300 mcg x 3 days Anemia Differential Diagnosis: Blood loss, Fe deficiency, megaloblastic, bone marrow suppression, meds, renal insufficiency, hemolysis Work-up: Complete history and physical, CBC, FE, TIBC, Ferritin, B12, haptoglobin, reticulocyte count, erythropoeitin level, serum folate, stool for occult blood (3), consider NGT Treatment: Nutritional deficiency: Replete iron, B12, folate GI bleeding: Consider upper and lower endoscopy Hemolyisis: Consider stopping offending medications, ?sickle cell, other hereditary problems including G6PD deficiency Marrow suppression: Consider bone marrow biopsy. Administer erythropoietin. Common transplant meds that cause anemia: azathioprine, chloramphenicol, hydralazine, hydrochlorathiazide, dapsone, neomycin, nitrofurantoin, phenobarbital, phenytoin, sulfasalazine, trimethoprim, rifampin, sulfonamides, streptomycin, tetracycline Thrombocytopenia Differential Diagnosis: Medications, heparin-induced, bleeding, hypersplenism (common in post-liver transplant patients with portal hypertension), fungal infection, sepsis, bone marrow suppression, aplastic anemia Work-up: Determine if pancytopenia present. Review medications, check for HIT, bleeding, infection work-up, consider bone marrow biopsy Treatment: Disease specific Nausea/Vomitting Differential Diagnosis: Medications, urinary tract infection, especially after renal transplant, gastritis, esophagitis, diabetic gastroparesis, small bowel obstruction, intraabdominal infection/sepsis Work-up: Review medications, Abdominal CT scan with po contrast, consider EGD Treatment: Nasogastric tube, foley, fluids. Can change cellcept to QID or decrease dose if no other cause found Diarrhea Differential Diagnosis: Medications, infection including c. dif, tube feeds. Work-up: Review medications, stool for culture, C. Dif, salmonella, shigella, yersinia, campylobacter, crytosporidium, microsporidium, ova and parasites. Treatment: Disease specific Rash/eruption/vesicles Differential Diagnosis: HSV1 and 2, herpes zoster, medications, cellulitis Work-up: History and physical, culture vesicle discharge Treatment Vericella Zoster- Valacyclovir 1 gram po TID x 7 days or acyclovir 5 mg/kg IV q 8 hrs x 7 days Oral Herpes : Valacyclovir 2 grams BID x 1 day or Zovirax cream to lesions 6 times a day. Hyperkalemia Differential Diagnosis: Calcineurin inhibitor toxicity, other medications including ACE inhibitors, K+ sparing diuretics, hyperglycemia, renal failure, rhabdomyolysis, diet Workup: Review meds, metabolic panel. Check EKG Treatment: If severe (>6.5), consider insulin, glucose, calcium, bicarbonate. Remember these are temporary solutions and K+ may rebound. Consider emergent dialysis, especially if there are EKG changes. Kayexelate with attending approval due to risk of bowel perforation. Decrease K+ in diet, consider lasix, consider Florinef 0.1 mg TIW to 0.1 mg BID for patients without hypertension, d/c ACE inhibitors, or other K+ sparing medications. Consider reduction in calcineurin inhibitor, correct hyperglycemia if necessary Hypertension Post-Renal Transplant The following guidelines should be applied in consultation with transplant nephrology. Immediate post-operative issues: Many renal transplant recipients will have immediate post-operative poor BP control due to volume overload. Often, allowing for a diuresis will assist in managing hypertension. Hypotension should be avoided because of the risk of prolonging ATN or thrombsis, hypertension (SBP >140 or DBP >90) does not improve renal function. Persistent SBP > 150 or DBP >95 should be treated with additional anti-hypertensive therapy. Always know what the patient was on to control blood pressure in the past. 1. Prior medications, Clonidine and beta-blockers should be continued post transplant to avoid rebound hypertension, unless they were previously being given at low doses. ACE inhibitors and ARBs should be held immediately post transplant because they can cause acute renal failure, anemia and hyperkalemia. They can be restarted as needed once the target tacrolimus or cyclosporine levels are lower, in the outpatient setting. 2. Patient is NPO. Metoprolol and hydralazine are good choices for patients who are NPO. Metoprolol is usually picked first, unless HR <70. Clonidine can be continued as a Catapress patch. 3. Cardiac Disease. Beta-blockers are the first choice in patients with a cardiac history, or multiple risk factors for cardiac disease. Metoprolol is the most common choice for a beta-1 selective drug, while carvedilol (better studied) and labetolol (cheaper) are good choices for combined beta and alpha blockade. Asthma, PVD, diabetes and CHF are no longer considered contraindications to use of beta-blockers. 4. Calcium Channel Blockers. In the absence of cardiac disease, dihydropyridine calcium channel blockers (amlodipine, felodipine and nifedipine) are the best first choice, because of data showing that they reduce the nephrotoxicity of calcineurin inhibitors (TAC and CSA). Diltiazem and Verapamil should be avoided in transplant patients because they interact with calcineurin inhibitors. If patients are on these pre-transplant, they should be switched to dihydropyridine calcium channel blockers and/or beta-blockers. 5. Patient has bladder outflow problems. Alpha blockers (such as doxazosin) are useful in men with bladder outflow problems. 6. Other drugs to avoid. Thiazide diuretics should be avoided post-transplant due to lack of efficacy in patients with renal insufficiency and possible volume depletion. Chronic use of Clonidine, Aldomet, Hydralazine and Minoxidil are third and fourth line choices, and usually are not necessary in patients with functioning renal transplant. If the patient is on clonidine pre-transplant, it can be tapered off in the outpatient setting. 7. Hypertensive emergencies (with symptoms of end-organ damage) should be managed in the ICU with continuous infusions such as Nipride. Again, **Hold all ACE Inhibitors and ARBs (Angiotensin receptor blockers) for 6-8 weeks after transplant due to their propensity to cause ARF and hyperkalemia in the patient with elevated Tacrolimus or Cyclosporine levels. ** Hyperlipidemia Post-Renal Transplant Hyperlipidemia is very common in the renal transplant patients and half of all transplant recipients are on a cholesterol lowering drug. The ALERT study showed that a statin can lower LDL by 32% and reduce the incidence of cardiac death and non-fatal myocardial infarctions. While we hold HMG-CoA reductase inhibitors (statins) at the time of transplant, we measure a fasting lipid profile at week 4 post op. At this time, statins are often restarted. Plasma levels of HMG-CoA reductase inhibitors are increased in renal transplant patients on cyclosporine and tacrolimus. It is prudent to use half the dose in transplant recipients and periodically monitor fasting lipid levels. New onset diabetes after transplant (NODAT) NODM is a major complication of transplantation and is a risk factor for renal allograft loss and independent of age, sex or race. USRDS database shows the cumulative incidence of NODAT in the United States is 9.1% at 3 months, 16% at 12 months and 24% at 36 months. Some of these patients may have been destined to develop diabetes even without transplantation, owing to risks from both genetic predisposition and age. Nevertheless, the incidence of new onset diabetes is fairly linear with time in patients on dialysis but increases disproportionately after transplantation. Low level blood glucose elevations can happen in many patients during steroid pulse therapy, but ongoing abnormal blood glucose levels must be noted and appropriate monitoring and education begun and treatment initiated as appropriate. XIII. Liver Transplantation--Background The problem The number of patients waiting for a liver transplant increased over the past decade from 3,955 in 1994 to 17,171 in 2003 while the number of patients undergoing liver transplantation increased from 3,574 in 1994 to 5,344 in 2003. There are currently 35,000 patients alive with a functioning liver transplant Indications 1) debilitating or life-threatening liver failure 2) early stage hepatocellular cancer when resection is not possible due to concomitant liver disease or tumor location. Categories of disease leading to need for transplantation 1) non-cholestatic cirrhosis 2) cholestatic cirrhosis, 3) metabolic disease 4) cancer, 5) biliary atresia 6) acute fulminant hepatic failure 7) miscellaneous Non-cholestatic cirrhosis Ongoing hepatocyte injury is the defining aspect of this diverse group of diseases. Cirrhosis is the ultimate result. Gastric and esophageal varices and ascites frequently develop as a consequence of portal hypertension as blood flow through the liver meets increasing resistance. Later stages of disease manifest synthetic dysfunction by coagulopathy, failure of bilirubin metabolism and excretion, malnutrition and hypoalbuminemia, fatigue, pruritus, hepatorenal syndrome, hepatopulmonary syndrome, and encephalopathy due to failure of the liver to adequately detoxify portal blood. Hepatitis C Most common reason for liver transplantation—up to 50% at some centers. Approximately four million people in the United States have chronic hepatitis C infection Viral infection occurs primarily via exposure to infected blood. Approximately 70% of infections will result in chronic hepatitis 10-15% of these patients will go on to develop cirrhosis. Concurrent alcohol use or co-infection with hepatitis B greatly increases the risk of progression to cirrhosis. Responsible for approximately 1/3 of hepatocellular cancers in the United States; approximately 25% to 30% of HCV-positive patients will develop HCC. Once cirrhosis is established the risk of HCC may is as high as 4% per year. Alcoholic Second most common indication for liver trasnplantation—25% Cryptogenic 3% percent of patients still undergo liver transplantation when the cause is not known Diagnosis of exclusion Autoimmune Constellation of signs and symptoms and the exclusion of all other causes. Skin rash and musculoskeletal complaints are common. Elevated ALT, AST, and bilirubin, but relatively normal alkaline phosphotase. Patients are more commonly female. Hepatitis B Approximately 300 million people in the world are infected, 250,000 die each year as a consequence. Effective vaccines available. The majority of acute infections in adults result in clearance of the virus and no sequelae. In up to 5% of patients, however, chronic infection will occur. Cirrhosis will occur in 15% of these patients. 20% of these patients will progress to decompensated cirrhosis requiring liver transplantation. 10% of patients with hepatitis B cirrhosis will develop hepatocellular cancer. Lamividine and hepatitis B immunoglobulin have allowed transplantation of these patients with good outcomes 1) The introduction of Hepatitis B immunoglobulin for prophylaxis against graft re-infection following transplantation for HBV has increased the overall survival rate to greater than 80% for 1 year and 65% for 3 years. 2) The high rate of re-infection after liver transplantation is probably due to the enhanced virus replication resulting from immunosuppression or direct stimulatory effect of steroid therapy on the steroid responsive enhancer region of the HBV genome. 3) Factor associated with a lower rate of re-infection include: pretransplant HBeAg negative assay, negative HBV DNA assay by non PCR technique, fulminant HBV, co-existent D virus infection 4) Passive immunoprophylaxis with HBIg is based upon the rationale that HBs antibodies will bind to and neutralize circulating virus. Many patients become re-infected if HBIg is stopped so long term passive immunoprophylaxis is required if HBIg is used as a sole agent to prevent re-infection. 5) Recurrence of HBV infection of the graft in patients on HBIg suggest (a) inadequate dosing schedule (b) the emergence of viral escape mutant which are typically mutations in the “a” determinant of the HBsAg. The emergence of these viral escape mutants appears to be temporally related and suggest that more effective anti-viral prophylaxis is required. 6) The presence of replicating virus in the serum prior to transplantation, as evidenced by a positive HBeAg assay or high titer HBV DNA, is associated with a higher re-infection rate despite HBIg. To combat this problem, active antiviral therapy has been instituted in most transplant centers to reduce viral replication. Lamivudine (Epivir, 3TC) is the most commonly used drug for this purpose. It is orally administered and is tolerated well. It has also been associated with the development of viral escape mutation (typically YMDD mutants) in the polymerase region of the viral genome. 7) To prevent recurrence a combination of Lamivudine begun preoperatively and HBIg begun perioperatively has been adopted as standard practice. In one study, there was no recurrence of HBV in 60 patients treated with this combination followed for a mean of 449 days. Non-alcoholic steatohepatitis (NASH) 3% of all transplants Obestity strong risk factor Cholestatic liver disease/cirrhosis Chronic biliary damage characterizes this group of diseases. Progressive impairment of bile flow leads to a cycle of obstruction and infection, which in turn exacerbates the biliary damage. Obstruction initially most often causes pruritis, which can be severe and debilitating, and later jaundice. Cholangitis may also result, producing fever and right upper quadrant pain, and even biliary sepsis. In general, the ongoing biliary damage predisposes the patient to the development of cholangiocarcimona, an aggressive malignancy that is generally a contraindication to transplantation. Primary biliary cirrhosis Progressive autoimmune disorder for which there is no effective medical therapy Fourth most common indication for liver transplantation Granulomatous destruction of the intralobar bile ducts Middle-aged women are most commonly affected. Positive AMA is the hallmark of the disease. Primary sclerosing cholangitis Unknown etiology, characterized by inflammatory fibrosis of the intrahepatic and/or extrahepatic biliary tree leading to diffuse structuring Approximately 60% of PSC patients have concomitant inflammatory bowel disease Cholangiocarcinoma will develop in up to 30% of patients. Caroli’s disesae Caroli’s Disease is a rare indication for liver transplantation caused by a genetic defect in a protein named PKHD1 that may be involved in hepatocyte growth. Segmental dilations of large intrahepatic bile ducts lead to chronic biliary stasis. Intraductal lithiasis is common and can be difficult to treat. Recurrent biliary obstruction may produce sepsis and intrahepatic abscesses. Biliary atresia Most common indication for liver transplantation in children Fulminant liver failure Liver failure within 8 weeks of the onset of symptoms in patients without pre-existing liver disease (except in the case of Wilson’s disease). Encephalopathy is the sine qua non of this disease. Causes: acetaminophen overdose (20%), cryptogenic liver failure (15%), idiosyncratic drug reactions (12%), hepatitis B (10%), and hepatitis A (10%). Other important causes include Budd–Chiari syndrome, herpes simplex infection, Epstein–Barr infection, paramyxovirus infection, Amanita poisoning, acute fatty liver disease of pregnancy, autoimmune hepatitis, and neonatal hemochromatosis. Wilson’s disease is unique in that fulminant hepatic failure can occur in these patients regardless of pre-existing disease. Deciding which patients with fulminant liver failure should undergo transplantation is one of the most difficult decisions in all of transplantation. King’s College criteria for poor prognosis: After Tylenol overdose: arterial pH less than 7.3 after volume resuscitation, or the combination of a PT greater than 100, creatinine greater than 3.3, and grade III or IV encephalopathy (arousable but unable to perform mental tasks, or worse). Poor survival after the development of fulminant liver failure after all other causes is best predicted by either a PT greater than 100 seconds or any three of the following: age less than 10 or greater than 40, interval between jaundice and encephalopathy greater than 7 days, bilirubin greater than 300 mumol/L, PT greater than 50 seconds, and a cause of either drug induced, nonA/nonB, or halothane induced hepatitis. Metabolic Diseases Second most common indication for liver transplant in childeren after biliary atresia Categories: 1) Diseases that cause structural liver damage leading to liver failure: a1-antitrypsin defi ciency, Wilson’s disease, tyrosinemia, urea cycle defects such as ornithine car bamoyltransferase (OTC) deficiency, galactosemia, glycogen storage disease IA and IV 2) Diseases that do not cause structural damage: familial hypercholesterolemia, primary oxalosis, protein C deficiency, hemophilia A or B). Malignancies Hepatoblastoma Hemangioendothelioma Hepatoma Early stage tumors have good prognosis with liver transplantation. Transplantation should be reserved for patients that are unresectable with early stage tumors Cholangiocarcinoma Contraindication for liver transplantation. Other Budd-Chiari Syndrome Occlusion of the main hepatic veins Presents acutely with right upper quadrant abdominal pain, hepatomegaly, and massive ascites. May be causesd by hypercoagulable states such as polycythemia vera, protein C deficiency, protein S deficiency, or antithrombin III deficiency. Prior to the development of cirrhosis, the treatment of choice is a side-to-side portacaval shunt to decompress the liver. Once patients have cirrhosis and secondary complications related to their liver failure, portal vein or inferior vena caval thrombosis, or a failed portacaval shunt, liver transplantation is best. Additional indications for Liver Transplantation TPN/Hyperalimentation induced Liver Disease Polycystic liver disease Large, unresectable hepatic adenomas due to concern for malignancy, bleeding, or pain. Intracranial Pressure Monitoring (ICP) in patients with fulminant hepatic failure Cerebral edema is a common cause of death in patients with fulminant liver failure. ICP monitoring serves as 1) a guide to therapeutic intervention and 2) a prognostic indicator. Goal is to keep ICP below 20 mmHg and CPP (MAP-ICP) above 70 mmHg to preserve perfusion to the brain. Interventions include mannitol, diuretics, vasopressors and dialysis. Patients in whom the ICP and CPP can not be controlled for prolonged periods of time should be considered for removal from the waiting list. Hemorrhage is the major risk of ICP monitors. Fatal hemorrhage occurred in 1% of patients with the epidural catheter, 5% of subdural and 4% of parenchymal catheters. Data from single center experience show that ICP monitors can be used safely and can improve management. Bleeding risk can be decreased by the use of recombinant factor VIIa as demonstrated in a recent study. Liver Allocation Each patient is assigned a number, termed the MELD (model of end-stage liver disease) score, based on three laboratory values: creatinine, international normalized ratio of the prothrombin time INR), and bilirubin. Prospective studies demonstrate this formula can accurately predict mortality from liver disease. Livers are allocated to patients with the highest MELD score. Patients with stage II hepatocellular cancer (2-5 cm lesion or no more than 3 lesions, the largest less than 3 cm) receive MELD points equal to the probablility of death on the waiting list of 15% within 3 months. This results in a significant number of trasnsplants for HCC. The decision not to grant points for small lesions reflects the low rate of metastases of these lesions. Larger lesions do not receive points due to the poor outcomes observed when transplanting patients with advanced disease. Treatment of Patients with Liver Failure Acute Liver Failure Patients with acute fulminant liver failure require urgent evaluation and consideration of listing for liver transplantation. Admission to the intensive care unit is mandatory with aggressive multisystem support. The proximate cause of death in most patients is sepsis or cerebral ischemia and care is directed toward preventing theese complications. Prophlactic antibiotics are genrally started. Prevention of cerebral ischemia is critical, and consists of maintaining the cerebral perfusion pressure (mean arterial blood pressure minus intracranial pressure) above 60 mmHg. This is accomplished by minimizing cerebral edema with hypoventiallation, fluid restriction, and mannitol as needed. Serum osmolality is maintained at approximately 325 msom/dl. Placement of an intracranial pressure monitor is controversial. No studies demonstrate improved outcomes with these devices, but they allow precise management of CPP and may be a useful guide to prognosis if the CPP is less than 60 mmHg for extended periods of time. Often these patients develop high output cardiac failure with low systemic vascular resistance requiring vasopressor support. When renal failure occurs, continuous veno-venous hemodialyis is recommended as it produces less circulatory stress than conventional hemodialysis. Patients in stage III or IV coma are intubated when there is concern regarding respiratory insufficiency or a risk of aspiration. Lactulose may be given by nasogastric tube to improve encephalopathy. Risk of bleeding is minimized with fresh frozen plasma and cryoprecipitate to decrease the INR below 1.5 and platelet infusions to keep the platelet count above 100. Contraindications Absolute and relative contraindications to liver transplantation have decreased over the past several years as our ability to successfully transplant sicker and more complex patients has improved. Absolute contraindications include advanced uncorrectable cardiac or pulmonary disease, severe irreversible pulmonary hypertension, irreversible neurological impairment, uncontrolled sepsis, and moist instances of extrahepatic cancer. Protocols exist for a number of diseases that were previously thought to be absolute contraindications for transplantation. These include human immunodeficiency virus (HIV) infection, advanced hepatocellular cancer, and cholangiocarcinoma. Interestingly, some of the most difficult problems to overcome do not relate to the operation but rather to the patients ability to follow the prescribed immunosuppressive regimens and take care of the liver. Included in this group is lack of social and economic support, patients who are actively using alcohol, and those with a history of non-compliance with medical care. Kings college criteria. Poor survival after Tylenol overdose is best predicted by either an arterial pH less than 7.3 after volume resuscitation, or the combination of a PT greater than 100, creatinine greater than 3.3, and grade III or IV encephalopathy (arousable but unable to perform mental tasks, or worse). Poor survival after the development of fulminant liver failure after all other causes is best predicted by either a PT greater than 100 seconds or any three of the following: age less than 10 or greater than 40, interval between jaundice and encephalopathy greater than 7 days, bilirubin greater than 300 mumol/L, PT greater than 50 seconds, and a cause of either drug induced, nonA/nonB, or halothane induced hepatitis. Liver transplant work-up History Physical examination Laboratory studies: complete metabolic panel, amylase, lipase, CBC, coagulation studies Chest X-ray Electrocardiogram Current hepatitis serological results (A, B, C) Viral serology: HSV), CMV, EBV, HIV, VZV Infectious serology: RPR, toxoplasmosis, rubella Tumor markers: alpha-fetoprotein (AFP), CA19-9 (if suspected cholangiocarcinoma or PSC) ABO and HLA typing CAT scan, MRI, or MRA of the liver for vessel patency, liver volume, and to rule out liver tumors. Dental evaluation Pap smear Mammogram in women over 40 PSA in men over 40 Consider: Upper GI endoscopy, pulmonary function tests with arterial blood gases, colonoscopy in patients over 40, cardiology evaluation with appropriate tests, psychiatric evaluation Outcomes Patient survival following cadaveric liver transplantation using brain dead donors: 93% at three months, 88% at one year, 80% at three years, and 74% at five years. Graft survival rates are 88% at three months, 81% at one year, 72% at three years, and 66% at five years Complications Hepatic Artery Compromise of the arterial flow to the graft occurs in 5-10% of patients and can be caused by hepatic artery thrombosis, stenosis, pseudoaneurysm, mycotic aneurysm, rupture, or compression by the arcuate ligament. All are serious complications that require urgent diagnosis and treatment. Treatment options include dilation, stenting, revision, or expectant management. HAT usually occurs within the first 2 months post-transplant and can be associated with one of three syndromes: (1) acute, massive hepatic necrosis leading to fulminant hepatic failure; (2) biliary tract ischemia leading to bile duct necrosis and leak; or (3) relapsing bacteremia with recurrent febrile illnesses due to cholangitis from biliary strictures. Nearly 75% of patients with HAT require retransplantation, although some grafts can be salvaged if HAT is recognized early and revascularization of the HA is urgently accomplished, so that screening Doppler ultrasound is recommended. Presentation, diagnosis and management differ depending on whether the thrombosis occurs less than or greater than one month after transplant. UNOS elevates patients with hepatic artery thrombosis within one week after transplantation to status 1 in recognition of the poor prognosis of this lesion. Almost all will present with increases in transaminase levels, while cholestasis is also very common. Risk factors include donor age greater than 60 and reconstruction of variant arterial anatomy. Patients with late thrombosis late HA risk factors include interposition grafts and donors dying of cerebrovascular accident. Complications include biliary ischemia, recurring cholangitis, and intrahepatic absesses. These complications require retransplantation in as many as 75% of patients. Hepatic artery stenosis occurs in approximately 4.8-11% of patients. Patients with this lesion are at markedly increased risk of biliary complications than control groups. Good results have been achieved with percutaneous radiologic treatment. Patient survival of 65% at four years following arterial revision has been achieved using either operative of percutaneous approaches. Arcuate ligamant syndrome occurs when the muscular and fibrous bands overlying the celiac axis compromise hepatic artery supply. This problem should be treated by reconstruction of the HA with an iliac artery conduit from the supraceliac or infrarenal aorta. Portal Vein Rates of portal vein thrombosis (PVT) after liver transplantation vary dramatically depending on the details of the transplant. In uncomplicated adult transplants, the rate is on the order of 1%. The presence of portal vein thrombosis prior to transplantation, use of split livers, use of portal vein conduits, and pediatric transplantation greatly increase the incidence to as high as 13%. Diagnosis is most commonly made by ultrasound. Percutaneous therapy including thrombolysis, venoplasty, and stent placement, for portal vein stenosis and thrombosis are often successful in the short term, but long term data is lacking. These options are particularly attractive when compared to the risks of operative revision of the anastamosis and possible thrombectomy. Biliary Tract Approximately 10-15% of patients will develop biliary complications after standard adult to adult cadaveric liver transplant. Split-liver and live-donor grafts have a higher incidence of biliary complications, as do grafts from nonheart beating donors. Complications inlude leaks from anastamotic sites, T-tube entry sites, strictures at the anastamosis or of the biliary tree, and obstruction from sludge, stones, or stents, and biliary fistula from stent migration. Anastomotic leaks usually present with bilious drainage from surgically placed drains or from the wound, or with signs of intraabdominal infection. Cholangiography through the T-tube or biliary stent can determine the site and severity of the leak. Patency of the HA should be confirmed by ultrasonography or angiography. A CAT scan should be obtained to rule out a subhepatic biloma. Rejection Acute hepatic allograft occurs in 30% to 75% of recipients, depending on the type of induction immunosuppression used. This is almost always cellular rejection, although humoral rejection can occur. Elevated liver function tests may include elevated transaminases, alkaline phosphatase, or bilirubin, alone or in combination. Rejection can be difficult to diagnose clinically as the differential diagnosis of abnormal liver function tests is broad, including preservation injury, ischemia, viral infection, bacterial cholangitis, bile duct obstruction, and recurrent disease. Therefore, a biopsy is usually obtained when acute rejection is suspected or any time the etiology of elevated hepatic function tests is in question. Other studies useful in determining the etiology of elevated liver function tests include cholangiography, duplex ultrasonography of the hepatic vasculature, and, if necessary, CT angiography with computerized reconstructions or hepatic arteriography. Acute rejection is histologically characterized by three features: (1) a portal mononuclear infiltrate (2) bile duct epithelial damage and infiltration by small lymphocytes and (3) subendothelial inflammation of portal or terminal hepatic veins (endothelialitis). Diagnosis of rejection requires at least two of these histopathological findings and biochemical evidence of liver damage. The diagnosis is even more assured if more than 50% of the bile ducts are damaged or if unequivocal endotheliitis is demonstrated. Risk factors for acute rejection include underlying liver disease (acute fulminant hepatic failure, hepatitis B, and autoimmune chronic active hepatitis), younger age of recipient, serum creatinine below 2.0 mg/dl, lack of edema, renal failure, or ascites, fewer HLA-DR matches, donor age, and cold ischemia time greater than 15 h. Acute rejection does not have a significant effect on patient or graft survival rates. This finding is in direct contrast to kidney transplantation where acute rejection episodes correlate with poorer graft survival rates. Chronic rejection is a progressive immunological process characterized by increasing cholestasis with an elevation in alkaline phosphatase, g-glutamyl transpeptidase, or 5’-nucleotidase and eventually bilirubin that occurs uncommonly in the current era of potent immunosuppressive drugs.. Histologically it is characterized by obliterative vasculopathy and loss of bile ducts (“vanishing duct syndrome” or “paucity of bile ducts”) involving more than 50% of the portal triads. Early signs of chronic rejection can include the subendothelial accumulation of foamy histiocytes with subsequent subintimal fibrosis and occlusion. Chronic rejection is often difficult to diagnose because arteries of the size involved with chronic rejection are not usually present on needle biopsies. Therefore, it has been shown that centrilobular ballooning degeneration and necrosis in the setting of previous acute rejection episodes and loss of bile ducts suggests chronic rejection. Chronic rejection usually develops following an unresolved acute rejection episode, after multiple acute rejection episodes, or slowly over many years in patients with a history of remote acute rejection episodes or no apparent episodes of acute rejection. Chronic rejection is not always irreversible, and bile duct regeneration can occur. Treatment of rejection with corticosteroids can be accomplished by a short course of intravenous methylprednisolone boluses (e.g., 250–1000 mg/day for 3–5 days) or by an increase in the oral prednisolone dose (e.g., 2 mg/kg/day) with a tapering schedule over 2 to 4 weeks. The only controlled study in liver transplant recipients showed that the administration of 1000 mg methylprednisolone followed by a 6-day taper from 200 to 20 mg/day is more effective and safe than 1000 mg of methylprednisolone for three consecutive days in the treatment of acute cellular rejection. The current incidence of steroid resistant rejection is 5% to 10% and is probably decreasing as immunosuppression improves. Treatment of steroid-resistant rejection is usually with OKT3 mAb or Thymoglobulin, but MMF and FK506 have both been used to rescue patients with steroid and OKT3 resistant rejection. Prior to initiation of OKT3 presence of anti-murine antibodies should be evaluated. Anti-murine antibodies should be checked weekly while on OKT3 or if inadequate response is observed. CD3 counts should be monitored daily to ensure a CD3 count (absolute #) < 25. If there is a persistent rise in CD3 count or if Cs3 count is greater than 50 increase OKT3 dose to 5 mg daily. XIV. Kidney Transplantation--Background Indications Kidney transplantation is indicated for end stage renal disease in patients who can tolerate the operation. There are cuurenly over 60,000 patients on the waiting list for kidney transplant among a dialysis population of 400,000. Kidney transplantation results in decreased mortality and improved quality of life and 50% decreased mortality as compared to dialysis. Disease processes that can be treated with transplantation include glomerulonehritis, chronic pyelonephritis, hereditary disease including polycycstic kidney disease, nephritis including Alport’s, tuberous sclerosis, metabolic disease including diabetes, hyperoxaluria, cystinosis, Fabry’s disease, amyloid, gout, and porphyria. In addition, obstructive uropathy, toxic insults, multisystem diseases including lupus, vasculitis, and scleroderma are treated successfully with transplantation A subset of disease will recur after transplant; most do not cause graft loss, but the following can in the listed percentages: FSGS—30%, Mesangiocapillary type I glomerulonephritis—20%, Hemolytic-Uremic Syndrome— 50%, Oxalosis—90%. In general, recurrent membranous nephropathy, IgA nephropathy, SLE, DM, Amyloidosis and Cystinosis do not cause graft loss. Patient Evaluation Patients should be referred to a transplant center when expected to require dialysis within 6 months to one year, and may be listed if the GFR is less than 20. Complete history and physical should include signs and symptoms of peripheral vascular disease, coronary artery disease, abdominal aortic aneurysm, IVC clot, hypercoagulable state, cancer, HIV, psychosocial issues (family support, substance abuse, cognitive deficiency), or a dysfunctional urinary excretory system. Medicaid generally pays for the transplant and so insurance is seldom an issue. Allocation system Kidney assignment is based on a point system as follows: Each year of waiting PRA greater than 80%: Less than 10 years old: 11-17 years old: Previous organ donor: No HLA mismatch: One mismatch: Two mismatches: 1 point 4 points 4 points 3 points 4 points 7 points 5 points 2 points Outcomes Projected 10 year graft survival rates for recipients of live donor transplants are outstanding. A halpo-identical sibling (2 haplo match) transplant has a 31 year expected half life, while non-perfect sibling transplants have a half life of 17-18 years. HLA matched (0 mismatch) deceased donor kidney transplants have a projected half life of 14 years, while a non-matched deceased donor transplant has a projected half life on average of 10 years. Older recipients of expanded criteria donor (ECD) kidneys can expect a shorter wait time for the kidney and a shorter graft survival on average. The ECD is a donor over the age of 60, or over the age of 50 with two of the following: CVA as cause of death, HTN history or creatinine greater than or equal to 1.5 at the time of death. Major obstacles to long-term patient survival after kidney transplantation include cardiac disease, infection and malignancy. Death with a functioning kidney transplant accounts for half of the graft losses after the first year. Of these, almost half of the patients die from cardiovascular disease. Thus, management of their cardiovascular risk factors is extremely important to long term patient and graft survival. Short term Complications Acute renal failure-Antibody mediated rejection, early cellular rejection, graft thrombosis, arterial or ureteral stricture, acute drug toxicity, urine leak. lymphocele with hydronephrosis Metabolic disturbances-Hyperkalemia, hypocalcemia, hypophosphatemia Early post-operative bleeding Early post-operative cardiac events Early post-operative infection Long term Complications Cardiac Disease Infection Malignancy (Lymphoma or PTLD, Skin cancers especially squamous cell carcinoma) Chronic allograft nephropathy Non-compliance Recurrent and de novo renal disease Hyperlipidemia Hypertension Osteoporosis Hematologic disorders (anemia, erthyrocytosis) Live donor considerations Donor should be left with better kidney No renal disease--GFR greater than 100 Counsel patient there is no significantly increased of developing ESRD, 3 in 10,000 mortality XV. Pancreas Transplantation--Background Indications Pancreas Transplantation is indicated for adult, Type 1 diabetics who are uremic (and thus also require a kidney transplant). In select cases, it is indicated for non-uremic type 1 diabetics (i.e. in the presence of hypoglycemic unawareness, severe metabolic complications, failure of insulin therapy). It is also an option in secondary diabetes (i.e. after traumatic or surgical loss of the native pancreas). Malignant pancreatic disease is a contraindication to transplantation. Categories Simultaneous Pancreas and Kidney Transplant (SPK): This is the preferred operation for uremic diabetics, although limited by organ allocation and availability issues. The kidney and pancreas are from the same donor (usually deceased, but living-donor kidney and partial pancreas grafts have been used) and implanted in one operation. One of the benefits of an SPK transplant is that serum Creatinine can be followed as a surrogate marker of pancreatic rejection. Rarely, a deceased-donor pancreas transplant can be coordinated with a simultaneous living-donor kidney transplant, resulting in a single recipient operation. Pancreas after Kidney Transplant (PAK): Here, the pancreas is transplanted after the kidney as a second operation. This is a good option for uremic diabetics with living kidney donors, as wait times for a solitary, deceased donor pancreas tend to be short. However, two operations and two rounds of induction immunosuppression are required and outcomes are not quiet as good compared to SPKs. Pancreas Transplant Alone (PTA): Non-uremic diabetics may be candidates for this operation. However, the benefits of being ‘insulin-free’ and normoglycemic must be weighed carefully against the risk of surgery and immunosuppressive therapy. Pancreas Transplant Work-up History Physical examination Social Work evaluation, psychiatric evaluation if indicated Laboratory studies: complete metabolic panel, amylase, lipase, CBC, coagulation studies Chest X-ray Electrocardiogram, Stress test (Cardiac catheterization if stress positive) Current hepatitis serological results (A, B, C) Viral serology: HSV, CMV, EBV, HIV, VZV Infectious serology: RPR, toxoplasmosis, rubella ABO and HLA typing Dental evaluation if needed Pap smear Mammogram in women over 40 PSA in men over 40 Colonoscopy if age over 50 Arterial evaluation (duplex, angiogram) if indicated. Complications Thrombosis – arterial or venous thrombosis occurs in about 5 to 12% of cases and usually results in graft loss. The rates are slightly higher for enteric vs. bladder drained grafts. Thrombosis rates can be lowered by careful donor and recipient selection, meticulous surgical technique and post-operative use of aspirin and low dose heparin Leak – leak rates range from 5 to 18% for bladder-drained grafts and 4 to 9% for enteric-drained. Bladder leaks can frequently be managed non-operatively (percutaneous drainage, foley catheter) whereas enteric leaks almost always require surgical revision; graft salvage is attempted, but not possible if there is generalized peritonitis. Bleeding – Bleeding usually occurs from the pancreatic graft, and the incidence is higher when anti-coagulation is used (15-30%). A requirement of more than 4 units of blood or hemodynamic instability should prompt reexploration. Pancreatitis – this can be from peripancreatic infections, reperfusion injury, or urinary reflus in bladder-drained grafts. Treatment depends on the cause (ie antibiotics, percutaneous drainage or abdominal wash-out) Outcomes One year graft survival is currently 85% for SPK, 79% for PAK and 78% for PTA. Benefits of a pancreas transplant include improved quality of life, greater life expectancy (for SPKs), protection of native and transplant kidneys from nephropathy, reversal of neuropathy, stabilization of advanced retinopathy and amelioration of early retinopathy. XVI. Dialysis Access--Background Scope of the problem: Over 20 million people in US have chronic kidney disease, and 400,000 people on dialysis, representing huge morbidity and cost in the United States. Patients on dialysis have very poor long-term survival which is markedly improved after kidney transplantation. Access planning Access planning should begin when the GFR is less than 30ml/min/1.73m2. This involves a transplant evaluation, preservation of veins for access, and pharmacologic interventions including management of expected electrolyte imbalances/mineral deficiencies (iron deficiency, hypocalcemia, dyslipidemias). Decisions should be made regarding the suitability of peritoneal or hemodialysis. Peritoneal Dialysis Peritoneal dialysis is done in the home by infusion of solution through an intraabdominal catheter. The solution dwells and removes electrolytes, BUN and creatinine, and is then removed. Requirements for this type of therapy include a stable, clean living situation, and the ability to manage the solutions at home. Prior abdominal surgeries with adhesions are a relative contraindication but this can be overcome in some patients. Advantages of hemodialysis include more gentle hemodynamics, no need to spend 3-4 hours in dialysis, and less fatigue. Disadvantages are the requirement that patients be able to understand and assist in own care, and the risk of infection and peritonitis requiring catheter removal. The catheter is placed in the pelvis. Laparascopy may be helpful in selected cases. The catheter can be used 4 weeks after placement. Complications include poor clearance necessitating replacement or removal, and peritonitis. It may be possible to treat through certain bacteria, but fungal infections generally require removal Hemodialysis Hemodialysis is the most commonly used treatment. Evaluation includes a detailed physical exam including Allen’s test, notation of peripheral vascular disease, congestive heart failure, diabetes, age, and stigmata of venous hypertension. In general, primary fistulas are best, with lower infection and greater long term patency when compared with grafts. Access should be placed as distally as possible. Advantages include reliability and minimal home care. Disadvantages are the need for 3-4 hours of treatment 3 times/week, and that most will fail eventually A number of complications require discussion. In the case of infection, ALWAYS determine if there is a pseudoaneurysm. If unsure on physical exam and ultrasound should be obtained. In the absence of a a pseudoaneurysm, antibiotics may be successful. If no resolution, can perform exclusion with removal of infected portion with jump graft at same sitting. Infected pseudoaneurysms should be managed in the operatating room. Options for proximal and distal control include dissection of graft or atery or touniquet. Thrombosed grafts can be thrombectomized on an elective basis, whereas thrombosed fistulas should be operated on within 24 hours. Always consider the possible reasons for thrombosis, including inflow obstruction, intragraft narrowing, outflow obstruction, and central stenosis. On table venogram is often useful in determining the cause. Steal is a very serious problem. It will present with numbness and tingling in fingers, and decreased pulse and capillary refill. Loss of strength is a late finding. This problem should be addressed immediately by narrowing or ligating the access. Severe edema is almost always caused by central stenosis, and so graft ligation does not usually help. It is important to obtain a central venogram in these cases. Bleeding will generally stop with single finger direct pressure unless there is an aneurysm, which can quickly turn into “blood on the ceiling” which can be difficult to control. Have OR ready as soon as possible. Options to stop bleeding include removing all dressings and applying direct pressure to hole, suturing the opening, compressing the arterial portion of graft above bleeding site, and the application of a blood pressure cuff above the site as a last resort XVII. Immunosuppression—Background A brief guide to transplant immunology The cellular events leading to organ allograft rejection are similar to those that occur against microbes and tumors. The immune system of the allograft recipient responds to transplanted tissue from a genetically non-identical individual of the same species as it would to a foreign invader. Overlapping mechanisms evolved to discriminate self from non-self by recognizing foreign antigens present on the invader. The most important of these foreign antigens in transplantation are the human leukocyte antigens (HLA) encoded within the major histocompatibility complex (MHC) on chromosome 6. T lymphocytes typically recognize foreign antigens as peptide fragments presented in association with self HLA molecules. In organ transplantation, however, the precise molecular nature of the target (alloantigen) is less certain. T cells in a variety of forms recognize most likely, non-self HLA. Despite these differences, the outcome is the same. If left unchecked, the immune response will destroy the foreign invader while leaving self intact. T lymphocytes play a central role in the specific immune response to foreign antigens. Antigen recognition is the initiating stimulus for subsequent T cell responses. When a helper T cell encounters antigen to which it is programmed to react, it can respond initially in one of three ways. The T cell can: (1) become activated to make soluble factors that induce and amplify the immune response, (2) become anergized or (3) undergo programmed cell death. The path taken depends on a number of factors, not the least of which is the molecular context in which the antigen is presented to the T cells. T cell activation (path 1) involves two phases: antigen presentation (input phase) and effector functions (output phase). T cell anergy (path 2) is an antigen specific and antigen induced unresponsive state. After the encounter that leads to anergy, the T cell is unable to respond to antigen upon subsequent stimulation. Exposure of anergic T cells to high concentrations of the T cell growth factor IL-2 may break anergy and allow the cells to subsequently respond to antigen stimulation. Apoptosis or programmed cell death (path 3) is crucial to limiting or abolishing the immune response to antigen. These three pathways represent the initial response of a T cell to antigen. In the absence of ongoing antigen presentation, activated T cells undergo apoptosis. Some of the progeny of T cell clones, which became activated, will develop into antigen-specific memory cells. ABO (human erythrocyte antigen system): with the rare exceptions of emergency adult and pediatric procedures, all solid organ transplants follow lines of classic blood group compatibility Human Leukocyte Antigens (HLA): The HLA system was initially defined serologically using antibodies derived from large scale screening of serum samples from multiparous women. The biologic significance of polymorphisms in MHC molecules is that it provides a mechanism of increasing the number of peptides that can be bound to antigen presenting cells (APC) and presented to T cells within a population. The theoretical advantage to a given species is that, with increasing polymorphism, there is a decreasing chance of encountering a pathogen against which all members of a population have a poor response and to which they may all succumb. The phenomenon of allograft rejection is a direct result of this advantage to the species of MHC polymorphisms. The class I HLA proteins, HLA-A, -B and -C, have a high level of polymorphism. Based on serologic reactivities, there are approximately 30 distinct antigenic specificities for the HLA-A locus, 60 for the HLA - B locus and 10 for the HLA -C locus. Using nucleotide sequencing, even more polymorphisms have been identified for each locus (more than 90 for HLA-A, 200 for HLA-B and 50 for HLA-C). Only the Class I HLA proteins A and B are routinely matched in the setting of renal transplantation, as HLA-C is a less important target of T cell recognition. Class II HLA proteins are also highly polymorphic. Due to their more limited distribution on cells, serologic classifications of the class II proteins are more difficult and sequence-specific oligonucleotide probes (SSOP) have been used. The HLA proteins that play the most important role in allograft rejection are A and B in class I and DR in class II. These are the three types of HLA molecules on which HLA typing in transplantation is based. Individuals have 2 copies of each of these three genes, hence there are 6 HLA antigens per donor or recipient. When discussing HLA sharing, haplotype refers to half of the full HLA profile in a given individual. One haplotype is inherited from each parent. For example, a donor sibling has the following tissue type: A21, A4, B12, B5, DR 4, DR10 because she inherited A21, B12 and DR4 from her mother and A4, B5 and DR10 from her father. If she were to donate to a one haplo-match sibling, she shares half of her HLA molecules (i.e., A21 B12, DR4) with that sibling. Siblings have a 50 % chance of being a one-haplotype match as shown in the example above. On average 25% of sibling pairs will be haplo-identical and 25% will be a two-haplotype mismatch. The clinical influence of matching for HLA in living related renal transplantation is clear. Recipients of a two-haplotype match sibling transplant enjoy a graft half-life of 25 years as compared with a 16 year half-life for a one haplotype match and these in turn have a better outcome than a zero haplotype match. While the one-year survival rates of sibling transplants are nearly identical regardless of the degree of HLA match, the differences in transplant survival are apparent with increasing time after transplantation. The role of matching in cadaveric transplantation is more controversial. The impact of matching for HLA-DR and B appear to be greater than matching for HLA-A. The impact of matching for HLA-DR appears to be most important during the first 5 months post-transplant. This may reflect the period of time when donor APCs are prevalent. Although the importance of HLA matching was most evident in the precyclosporine era, it is still relevant. In the Collaborative Transplant Study of Opelz et al., of approximately 10,000 patients treated with cyclosporine, there was a 17% better 1 year graft survival rate in recipients matched for HLA -B and -DR antigens compared with those mismatched for all four -B and -DR antigens. The current UNOS policy involves sharing of zero mismatch (0 MM) cadaver kidneys nationwide. All other degrees of mismatching (1-6 mismatches) are distributed within the local Organ Procurement Organization (OPO) based on time on the waiting list, ABO compatibility and other factors such as the location of donor hospital. In addition, immediately prior to transplant, a serologic crossmatch is performed. The lab mixes donor cells with recipient serum to look for the presence of preformed antibody in the recipient against donor HLA antigens, which would lead to hyperacute rejection mediated by the humoral immune system. The crossmatch is positive if the recipient has pre-formed antibodies against donor HLA molecules. Therefore, if the crossmatch is positive, the kidney is then offered to the next acceptable recipient. Prior to transplantation, patients on the transplant list have a monthly PRA (panel reactive antibody) assessment that determines the likelihood that patient will have a positive crossmatch. Patients' sera are incubated with a series of B and T cells from a panel of donors selected to represent the HLA specificities. Patients who are highly sensitized to non-self HLA antigens due to blood transfusions, prior rejected transplants, or pregnancies are more likely to have a positive crossmatch to a potential donor and may wait longer for a transplant. Steroids Mechanism of Action: Alter the transcription and translation of several genes responsible for cytokine synthesis. They inhibit T-cell activation by blocking IL-1, IL-6, IL-2, and IFN synthesis. They also have local antiinflammatory effects. Primary Use: Corticosteroids are used as part of many induction and maintenance immunosuppressive regimens and are usually the first line treatment for allograft rejection. Many protocols now incor porate early corticosteroid withdrawal in order to avoid their long-term side effects. Advantages: Reliable immunosuppression Disadvantages: Side effects include Cushingoid features (moon facies, acne, centripetal obesity, striae), hypertension, increased appetite and weight gain, hyperglycemia, osteoporosis, avascular necrosis, posterior lenticular cataracts, growth retardation in children, poor wound healing, thinning of the skin with increased fragility and ecchymoses, pancreatitis, peptic ulceration, colonic perforation, steroid-induced hyperactivity or psychosis, and predisposition to infectious and malignant complications. Antimetabolites Mechanism of Action: Mycophenolate mofetil (MMF) is rapidly converted to the morpholinoethylester of mycophenolic acid (MPA), which inhibits inosine monophosphate dehydrogenases. This blocks proliferation of T and B lymphocytes and inhibits antibody formation and the generation of cyto toxic T cells. MPA also downregulates the expression of adhesion molecules on lymphocytes. Other drugs in this category include azathioprine, a purine synthesis inhibitor, and cytoxan, an alkylating agent. Their use has been supplanted by MMF. Primary Use: As an adjunct to primary immunosuppression to decrease the rate of acute rejection. Most of our patients will be on this drug. Dosing and Pharmacokinetics: MMF can be administered orally or intravenously. Starting dose is 1 gm bid (1.5 gm bid in African American renal recipients). Advantages: Decreases incidence of acute rejection Side Effects: Nausea, diarrhea, gastritis, leukopenia, anemia, and viral infections. Generally side effects are dose dependent. Diarrhea and nausea are treated by spacing the dose from 1 gm bid to 500 mg QID. Dose can be reduced to 500 tid, then bid, then 250 mg tid, then bid. Often patients over the acute surgery will tolerate increasing dose. Calcineurin Inhibitors Mechanism of Action: Cyclosporine (CsA) and tacrolimus (FK506) bind to immunophilins and inhibit calcineurin activity necessary for the transcription of genes that activate T-cells including IL-2, IL3, IL-4, and IFN. Sensitized cytotoxic and helper T cell generation is inhibited. Primary Use: Primary immunosuppression for kidney, liver, and pancreas patients. We have been using FK506 initially and switching to rapamycin after wounds heal. Dosing and Pharmacokinetics: Neoral is a microemulsion formulation of cyclosporine which we prefer due to greater bioavailability. Levels drawn two hours after administration (C2) may allow lower incidence of rejection without increasing side effects. Target level is 1000 ng/ml. FK506 is usually started at 0.07 mg/kg/day for the first month with target levels of 5 to 15 ng/ml. Advantages: Potent immunosuppression and extensive experience with these drugs. Side Effects: Both drugs are nephrotoxic and likely decrease graft survival over time. Cyclosporine: hypertension, hyperkalemia, hyperuricemia and gout, gingival hypertrophy, hirsutism, neurological abnormalities (tremors, seizures, hyperactivity, insomnia, confusion, depression, somnolence), hyperglycemia, hypomagnesemia, hypercholesterolemia, hypertriglyceridemia, hepatotoxicity, and the development of de novo hemolytic uremic syndrome. FK506: Neurological complications (headaches, tremors, paresthesias), and hyperglycemia. Hypertension and hirsuitism are more commonly associated with Cyclosporine and neurological side-effects and hyperglycemia with FK506. Antibodies ATGAM (Horse antithymocyte globulin) and Thymoglobulin (rabbit antithymocyte globulin) Mechanism of Action: Polyclonal antibodies that cause rapid decline in circulating T- and B lymphocytes due to apoptosis, complement dependent lysis and opsonization of lymphocytes with subsequent removal in the reticuloendothelial system. Primary Use: Induction therapy (rejection prophylaxis) and rejection. We use thymoglobulin almost exclusively Dosing and Pharmacokinetics: ATGAM 10 to 30 mg/kg/day IV for 3 to 14 days. Thymoglobulin is dosed 1.25-1.5 mg/kg/day. Pretreatment with steroids, Tylenol, benedryl decreases the severity of side effects. Advantages: Extremely potent immunosuppressive agents almost always effective in reversing rejection. Side Effects: Fever, chills, nausea, vomiting, diarrhea, athralgias, headache, myalgias, rash, pruritus, urticaria, phlebitis, leukopenia, thrombocytopenia, and predisposition to viral infections and posttransplant lymphoproliferative disorder. OKT3 Mechanism of Action: Monoclonal murine antibody that reacts with the T3 recognition complex on the surface of mature T lymphocytes and blocks the recognition of class I and class II antigens, resulting in the inhibition of the generation and function of effector T cells. Its primary mechanism of action in vivo involves the binding of OKT3 to circulating T cells with increase in the opsonization and destruction of these cells by the reticuloendothelial system. OKT3 also acts by modulation of the T3 antigen recognition complex of circulating T cells and by inhibiting the function of sessile T cells. Primary Use: Dosing and Pharmacokinetics OKT3 is administered intravenously at a dose of 2.5 to 5 mg/day for 3 to 14 days posttransplant for rejection prophylaxis, to reduce CNI exposure, and for the treatment of steroid resistant rejection. CD3 levels and antimurine antibody titers are monitored with goal to keep the CD3 <25. Advantages: Potent and reliable immunosuppression Side Effects: Side effects are most commonly seen with the first two doses and include fever, chills, diarrhea, headache, nausea, vomiting, dyspnea, wheezing, pulmonary edema, tachycardia, hypotension, aseptic meningitis, seizures, and coma. These side effects result from the release of tumor necrosis factor (TNF), IFN-γ, and IL-1. This symptom complex can be reduced by pretreatment of the patient with high-dose steroids, acetaminophen, diphenhydramine hydrochloride, and indomethacin. Between 15% and 40% of patients develop antimurine antibodies. Basiliximab (Simulect) Mechanism of Action a chimeric anti-CD25 mAb bearing the murine variable IgG2a antigen-combining site and IgG1 human constant regions. The advantage over murine antibodies is that it does not have the xenogeneic murine Fc region structures, but retains the variable region-combining site of the murine antibody and therefore does not stimulate a host antimurine immune response. The chimeric antibody binds to the high-affinity IL-2 receptor (IL-2R) and prevents binding of IL-2 to the receptor and blocks IL-2-driven proliferative responses, the duration of which is dose dependent. It has a mean half-life of 1 to 2 weeks. Basiliximab is administered intravenously at a dose of 20 mg at the time of transplant and 4 days later. This regimen produces a 30- to 45-day blockade of IL-2R expression in adult patients. Basiliximab is used primarily for rejection prophylaxis. No adverse side effects compared to placebo have been reported. Primary Use: Induction therapy for kidney or liver transplant recipients. Is not use as a treatment for rejection Dosing and Pharmacokinetics: 20 mg IV x 1 intraoperatively and 20 mg IV x 1 on POD 4 Advantages: Minimal side effects. Lover incidence of infectious complication post transplant than seen with ATG or OKT3. Side Effects: Potential for hypersensitivity reactions Daclizumab (Zenapax) Mechanism of Action: Humanized monoclonal antibody that is a human IgG1 containing the antigen-binding regions of a murine anti-CD25 Ab that binds to the high-affinity IL-2R in a similar fashion to basiliximab. Primary Use: Rejection prophylaxis Dosing and Pharmacokinetics administered intravenously in a dose of 1 mg/kg before transplantation and then every 2 weeks for a total of five doses. Its effects on IL-2R suppression last up to 10 weeks and is used primarily for rejection prophylaxis. Alternative dosing strategies of 2 mg/kg q 14 days x 2 doses have also been sown to be effective Advantages: Minimal side effects. Lover incidence of infectious complication post transplant than seen with ATG or OKT3 Side Effects: Minimal (rare anaphylaxis on re-exposure) Alemtuzumab (Campath-1H) Mechanism of Action: (C-1H) is a humanized, recombinant anti-CD52 monoclonal antibody that results in profound depeletion of mature T cells, and to a lesser degree B cells and monocytes for over a month. Primary Use: Rejection prophylaxis and long-term therapy Dosing and Pharmacokinetics: It is administered intravenously at the time of transplant and post-transplant at a dose of 0.3 mg/kg or at a fixed dose of 30 mg for one to three doses Advantages: Simple dosing regimen Side Effects: Hypersensitivity or infusion reactions Mammalian Target of Rapamycin (MTOR) Inhibitors Mechanism of Action: Rapamycin (Sirolimus) is a macrolide antibiotic that binds to FK binding protein isoform12, inhibiting TOR and the signal III pathway which blocks T cell activation and proliferation after cytokine stimulation and costimulation interactions. Primary use: Primary immunosuppression in most kidney and liver patients. Generally begun more than one month after transplant after wounds are healed. Dosing and pharmacokinetics: 1 to 5 mg po qd posttransplant. Half-life is 62 hours. Levels are monitored not more than every 3 days with target trough levels of 10 ng/mL in first 6 months. Advantages: Potent immunosuppression with little or no nephrotoxitcity. May allow improved renal function over time. Side effects: Anemia, leukopenia, thrombocytopenia, elevated serum cholesterol and triglycerides, gastrointestinal side effects, pneumonitits, oral ulcerations (especially if levels are too high), poor wound healing including lymphoceles. Error! XVIII. Infections More than half of transplant patients will have at least one significant infection, the majority being bacterial. Infections occurring in the first month are usually bacterial or fungal and related to the surgical procedure. From 1 to 6 months after transplant, viral infections including CMV, HSV, and EBV are most common. After 6 months, the risk of infection is low. Keep in mind that many of our patients have prolonged hospitalizations are will acquire resistant infections. Best to start with broad spectrum antibiotics and aggressively narrow them as culture results return. Bacterial infections Risk factors for the development of bacterial infections include length of operation (>12 h), multiple abdominal operations, retransplantation, pretransplant bilirubin 12 mg/dl or more, and intraoperative transfusions. The most common diagnoses include wound infections, intraabdominal infections (peritonitis, hepatic and extrahepatic abscesses, and cholangitis), pneumonias, urinary tract infections, central venous catheter infections, and bacteremias. The diagnosis of bacterial infections depends on their site, but involves cultures (blood, urine, sputum, wounds, drains, bile), ultrasonography, CAT scan, cholangiography, angiography, liver biopsy, CXR, bronchoscopy and bronchoalveolar lavage, and open lung biopsy. Antibiotic therapy is dictated by the organism and its antibiotic sensitivities. Keep in mind that many of our patients have frequent hospital admissions and resistant organisms. It is better to be aggressive with broad empiric coverage initally and then narrow the choice based on culture data. In many intraabdominal infections, fungal organisms will also be cultured and require appropriate antifungal therapy. Similarly, directed therapy of the infection will depend on its etiology and location. Superficial wound infections require drainage and local wound care. Subhepatic or subphrenic fluid collections, hematomas, and abscesses, as well as intrahepatic abscesses, usually can be effectively drained percutaneously, but may require operative drainage. Associated bile duct leaks should be excluded by cholangiography and, if present, hepatic artery thrombosis ruled out. Bacterial pneumonias and urinary tract infections usually respond to antibiotic therapy. Infected central venous catheters should be removed and antibiotics administered. Viral infections The most important viral infections after liver transplantation include CMV, EBV, HSV, and varicella zoster virus (VZV). These are generally reactivation infections caused by immunosuppression as opposed to de novo infection. These viruses are also potentially oncogenic. Other viral infections that can occur posttransplant include HHV6, HHV-7, HHV-8, papovavirus and adenovirus. CMV infection is the most important viral infection in transplant recipients. Use of valgancyclovir prophylaxis has dramtically reduced the incidnce of CMV disease, but the drug can cause leukopenia and anemia, both of which can be severe. We typically prophylax for 3 months unless the donor is CMV positive and the recipient CMV negative, in which case we give 6 months of therapy. CMV most commonly occurs in the first 3 to 6 months after transplant, with a peak incidence at 4 to 6 weeks. The classic clinical signs and symptoms include fever, malaise, myalgias, athralgias, and leukopenia. CMV may involve several organ systems, resulting in pneumonitis; ulceration and hemorrhage in the stomach, duodenum, or colon; hepatitis; esophagitis; mononucleosis; retinitis; encephalitis; glomerulopathy; and pancreatitis. The greatest risk of CMV infection occurs in seronegative recipients of organs from seropositive donors (D+R-). CMV PCR is the first test to diagnose infection, however it is critial to remember that invasive tissue infection can occur in the absence of serum CMV. It the appropriate clinical context biopsy should be performed. HSV infections usually occur during the first 6 weeks after transplantation and can be primary or reactivation infections due to HSV-1 or HSV-2. HSV-1 infections are usually mucocutaneous infections in and around the oral cavity, but esophagitis, hepatitis, encephalitis, pneumonitis, and ocular HSV infections can occur. HSV-2 can cause penile vesicular lesions, cervicovaginitis, and proctitis. The diagnosis can be made by physical examination, endoscopy and biopsy, liver biopsy, bronchoscopy, and culture of vesicular lesions. Serology is rarely helpful. Intravenous or oral acyclovir is effective therapy in most patients. EBV infection occurs in 5% to 10% of liver transplant recipients as a primary or reactivation infection and causes a spectrum of post-transplant lymphoproliferative diseases (PTLD). The incidence of PTLD ranges from 0.85% to 20% depending on age, type of transplant, degree and type of immunosuppression, and EBV serology, and may be localized or disseminated with high mortality. Treatment usually involves reduction or withdrawal of immunosuppression as first line therapy along with ganciclovir antiviral therapy. Other treatment modalities include interferonalpha, cytotoxic chemotherapy, anti-B-lymphcyte antibodies, and humanized anti-CD20 monoclonal antibody. VZV infections occur as primary or reactivation infections. Primary VZV infections in immunosuppressed patients can be severe, causing pneumonia; gastrointestinal tract ulceration and hemorrhage; and severe central nervous system, skin, and eye involvement. causing leading to rapid dissemination and death. Reactivation VZV (zoster) infections occur in about 7% to 10% of patients, months to years after transplantation. Visceral dissemination in these patients is rare. Disseminated VZV is treated with intravenous acyclovir along with a reduction in immunosuppression. Zoster may be trated with intravenous or oral acyclovir depending on the severity of the disease. In patients who have never had chicken pox, a history of recent exposure should be treated immediately with varicella zoster immune globulin, and acyclovir should be started at the earliest sign of disease. Fungal Infections Most fungal infections occur within the first month after transplant. Common organisms include Canidida species, Aspergillus, Mucormycosis and Cryptococcus. Candida species other than albicans are becoming increasingly common, and antifungals should be shoud be chosen with care as many will be resistant to fluconazole. Prophylaxis with nystatin or clotrimazole is effective in preventing oral candidiasis (thrush) and eosphagitis posttransplant, but is less effective in preventing invasive fungal infections. Oral fluconazole has been shown in a randomized, double blind, placebo-controlled trial to reduce fungal infections in liver transplant recipients and to be superior to nystatin in decreasing posttransplant fungal infections. Fluconazole is now used in many centers, but a randomized placebo-controlled trial showed the broad-spectrum triazole-itraconazole oral solution safely reduces the risk of fungal infections and may be superior because of its greater activity against Aspergillus species. Protozoal Infections Pneumocystis carinii causes dyspnea, fever, and cough associated with diffuse bilateral interstitial pulmonary infiltrates. The diagnosis is usually made by the identification of the organism using the methenamine silver stain on bronchoalveolar lavage fluid or by transbronchial or open-lung biopsy. Treatment with trimethoprimsulfamethoxazole or pentamidine is usually effective. Trimethoprim-sulfamethoxazole prophylaxis has essentially eliminated pneumocystis infections in transplant recipients. XIX. Operative Procedures Deceased Donor-Standard Technique A midline incision is made from the suprasternal notch to the symphysis pubis including a sternotomy. In obese donors, a cruciate incision made at the level of the umbilicus may improve exposure. Inspection of the abdomen to exclude malignancy or other disease process that would preclude organ retrieval is followed by inspection of the liver for size and steatosis. Fatty livers have a characteristic yellowish hue, rounded edges and tend to be large. Biopsy is taken for frozen section if an abnormality is suspected. Early control of both the supraceliac and infrarenal aorta in case the donor becomes unstable allows for rapid cannulation and aortic cross-clamping. Mobilization of the cecum, ascending colon and small bowel mesentery exposures the retroperitoneum. The aorta is looped at the bifurcation and the supraceliac aorta is controlled. Next, the gastrohepatic ligament is incised and the porta hepatis encircled. A replaced left hepatic artery arising from the left gastric artery (17%) will be palpable in the gastrohepatic ligament, whereas a replaced right (20%) will be palpable dorsal to the bile duct. Isolation of the proper hepatic artery is followed by identification and ligation of the gastroduodenal artery. Dissection of the common hepatic toward the aorta will identify the splenic artery. Continued dissection reveals the celiac trunk and aorta. Mobilization of the portal vein and identificaion of the bile duct copmlete the portal dissection. Division of the common bile duct close to the head of the pancreas allows irrigation of teh biliary tree through an incision in teh gallbladder. Next, a site is selected for placement of the portal infusion catheter, either the SMV, which can be identified above and to thr right of the SMA, or the IMV, found lateral to the ligament of Trietz. Removal of kidneys and pancreas requires further dissection of these organs. Freeing of the spleen, inferior border, and superior border of the pancreas in the warm facilitates its removal. Heparinization is followed by aortic and portal cannulation. At crossclamp, the supraceliac aorta is occluded, the vena cava is vented in the right chest, University of Wisconsin solution is infused rapidly, and the entire abdomen is packed in ice. Incision of an aortic patch around the celiac trunk facilitates removal and preservation of the arterial supply of the liver. Replaced right or left arteries are carefully preserved. The portal vein is transsected, the infrahepatic cave is transsected just above the renal veins, and the liver is removed. The SMA is then divided at the aorta to facilitate removal of the pancreas. Identification of both ureters allows safe removal of the kidneys. Deceased Donor-Donation after Cardiac Death Organs from donors after cardiac death (DCD) or non-heart beating donors and are increasingly used to expand the donor pool. Rates of primary non-function, 1 year graft loss and biliary complications are slightly higher with these grafts compared with standard donors. Center-specific protocols dictate patient preparation. Incision is after five minutes of asystole. A midline incision allows immediate packing of the abdomen with cold slush. Opening of the chest is surgeon-dependent. Expeditious dissection of the aorta allows perfusion of the abdominal organs. Venting is through the right atrium or IVC. Cross clamp of the thoracic or supraceliac aorta follows. The organs and abdominal contents are carefully inspected during the flushing procedure. Upon completion of flushing, the procurement can then proceed using one of the techniques already described. Deceased Donor Liver Transplant A bilateral subcostal skin incision with a midline extension to the xiphoid process is used. The falciform ligament is divided cephalad until the anterior surface of the suprahepatic vena cava is identified. The left lobe is mobilized by dividing the left triangular and coronary ligaments and the gastrohepatic ligament. The hilar dissection begins with division of the cystic duct and artery. The left and right hepatic arteries are divided close to the liver to provide adequate length for a branch-patch anastamosis to the donor HA. The HAs are then dissected free proximally to the bifurcation and a length of the common HA is isolated for subsequent clamping. If the HA is unsuitable for anastamosis at this point, further dissection to or beyond the takeoff of the GDA may be necessary. The tissue posterior to the HA is divided, exposing the anterior aspect of the PV, which is mobilized distally to the bifurcation and proximally to the head of the pancreas. The lymph node tissue inferolateral and posterior to the CBD is divided, and the posterior aspect of the PV is identified. An opening anterior to the PV and posterior to the CBD is then made, leaving much of the surrounding tissue adjacent to the bile duct intact to avoid its devascularization. The CBD is ligated adjacent to the liver and divided. The infrahepatic vena cava is then isolated. Mobilization of the right lobe of the liver is performed by dividing the right triangular and coronary ligaments. The liver is mobilized off the interior vena cava by dividing all the hepatic veins entering the posterior aspect of the liver. Only the left, middle, and right hepatic veins are left in place. A clamp is placed across the hepatic veins and the confluence of the veins opened. The donor infrahepatic vena cava is oversewn on the back table, eliminating one anastomosis, and the donor suprahepatic vena cava is sewn end-to-end to the confluence of the recipient hepatic veins. Venovenous bypass is not required. In addition, there is significantly less blood loss as a result of avoiding dissection posterior to the vena cava where bleeding from retroperitoneal collaterals can be encountered. The suprahepatic vena caval anastomosis is performed by sewing the donor cava to the confluence of the hepatic veins while infusing 1 l of cold lactated Ringer’s solution through the PV cannula. This flushes the liver of UW solution, which is high in potassium and contains heparin. It also flushes air from the liver and reduces the risk of air emboli. The portal venovenous bypass cannula is clamped and removed from the recipient PV that is clamped. The PV anastomosis is completed end-to-end with a loosely tied corner (“growth stitch”) to allow expansion of the PV after unclamping. If the PV is thrombosed, a long donor iliac vein graft can be sewn end-to-side to the SMV, which is isolated in the mesentery of the transverse colon. It is tunneled retrocolic, posterior to the antrum, and anterior to the pancreas for anastomosis to the PV. The bile duct is usually reconstructed as an end-to-end choledochocholedochostomy using interrupted absorbable sutures. Traditionally this has been performed over a T-tube brought out through a separate choledochotomy. However, because 10% to 15% of patients develop a bile duct leak after T-tube removal, necessitating an ERCP and nasobiliary drain or stent placement, some centers have eliminated the use of T-tubes with comparable results. If the recipient bile duct is not usable secondary to disease (e.g., PSC or large periductal varices) or there is a marked size discrepancy with the donor duct, an end-to-side Roux-en-Y choledochojejunostomy should be performed. A small 5-Fr. feeding tube is placed through the anastomosis and brought out through a tunnel in the Roux loop. The anastomosis is done as a single-layer anastomosis using absorbable suture. Drains are placed and the abdomen closed. Deceased donor kidney transplant Native kidneys are not usually removed. Transplant incisions are made from the pubis and extend two fingerbreadths medial to the anterior superior iliac spine. The retroperitoneal space is entered at the lateral border of the rectus where there is no posterior sheath and the peritoneum is reflected medially. In males the cord is preserved, in females the round ligament is generally ligated. Either kidney can be placed on either side. Placement into the right is usually easiest because the vein is more lateral to artery and easier to see and sew. In patients that are candidates for pancreas transplantation, kidneys should be placed on the left side to leave the right side open for pancreas transplant. Deceased donor pancreas transplant Midline incision, intraperitoneal placement Pancreas graft prepared by removing spleen, trimming graft duodenum and sewing donor iliac artery ‘Y’ graft to splenic artery and superior mesenteric artery; venous outflow via portal vein. Arterial inflow is derived from the recipient iliac artery. Venous drainage can be systemic (into iliac vein) or portal (into superior mesenteric vein). There are no differences in outcomes with either technique; systemic drainage is associated with hyperinsulinemia, which may theoretically cause the metabolic syndrome. Portal drainage is a useful option in patients with prior transplants in the pelvic area. The graft duodenum can be drained into the bladder or small bowel (with or without a Roux-en-Y). With bladder drainage, one can measure urinary amylase (levels fall with acute rejection); however bladder drainage is associated with several adverse effects, such as dehydration, metabolic acidosis, hematuria, cystitis and graft pancreatitis. Most centers now prefer enteric drainage, as rates of rejection are low with current immunosuppressive therapy. Wrist fistula (Cimino) Incision over head of radius between artery and vein, closer to artery Dissect vein first to ensure adequate caliber and quality Take vein down as far as possible toward wrist and transsect or swing up for end to side Dissect artery and perform anastamosis Forearm loop Incision just below antecubital crease Dissect any suitable vein Unlikely to find vein below biceps aponeurosis Dissect brachial artery above bifurcation Beware of early bifurcation Antecubital fistula Best on thin persons Brachiocephalic fistula If can’t follow vein up arm, unlikely to mature Basilic vein transposition Dissect out basillic vein from antecubital fossa toward axilla Tunnel under skin to meet brachial artery Sew primary anastamosis Upper arm graft Dissect vein 2/3 up upper arm Dissect artery above antecubital fossa Place tunnel as far lateral as possible Leg graft Considerations Higher infection rate Uncomfortable for dialysis Wound complication rate Peripheral ischemia Operation Anastamosis to saphenous Lecture Series 1) Liver Transplantation: Indications, evaluation of recipient and donor, operations, post-operative management 2 2) Kidney Transplantation: Indications, evaluation of recipient and donor, operations, post-operative management 2 3) Pancreas Transplantation: Indications, evaluation of recipient, the operation, post-operative management 1 4) Immunosuppression: Transplant immunobiology anti-rejection therapy 1 5) Post Transplant Complications: Infectious disease, cancer, rejection, drug toxicity, other 1 6) Dialysis and Dialysis Access 1 7) Miscellaneous Issues: Histocompatbility, Transplant Psychosocial and Financial Issues, Non-heart Beating Organ Donors, History of Transplantation, Transplant Pathology, Organ Donation and Brain Death, practical patient management issues: drug interactions, common side effects, management of acute events (bleeding, rejection, etc.) 1 XXII. Appendicies a. Drug Interactions The potential exists for drug interactions when numerous medications are given that are metabolized through similar pathways. The interaction may result in enhanced efficacy of the intended drug with a potential to lead to toxicity or reduced efficacy of the intended drug which may lead to rejection in the transplant setting. Both calcineurin inhibitors (CNI’s) cyclosporine and tacrolimus as well as sirolimus share similar metabolic pathways. All three agents are substrates of the cytochrome P 450 3A (CYP3A) isoenzyme system and P-glycoprotein.. Two types of interactions usually occur with medications metabolized via the CYP enzyme system, inhibitory interactions and inducing interactions. Enzyme inhibition occurs when there is enzyme inactivation or mutual competition of substrates at a catalytic site. This usually results in an inhibition of drug metabolism leading to increased serum concentrations, increased trough concentrations and a prolonged half life. Enzyme induction interactions occur when there is increased synthesis or decreased degradation of CYP enzymes. This can lead to decreased plasma concentrations of drug and a decreased pharmacodynamic effect. In addition to the numerous pharmacokinetic interactions that may occur with the CNI’s there exists the possibility for pharmcodynamic interactions as well. A pharmacodynamic interaction exists when the concomitant use of a drug results in an increase in the immunosuppressive effect or an increase in the toxicity of the CNI. The addition of nephrotoxic agents such as amphotericin B, aminoglysides (gentamicin, tobramicin, amikacin) and non steroid anti inflammatory agents (naproxen, ibuprofen, ketorolac) may potentate the know nephrotoxic effects of the CNI’s. Non-nephrotoxic antifungals such as caspofungin or micafungin should be thought of as alternatives for treatment of fungal infections in transplant recipients on a CNI based regimen. Acetaminophen should be the over the counter pain medication of choice in transplant recipients as it is devoid of nephrotoxic effects. Table 1 provides detailed information on the management of pharmacokinetic drug interactions in the transplant recipient. Table 2 is a list of herbal preparations that should be avoided in transplant recipients due to their potential for immunomodulatory effects or drug interactions. Drug Effect on enzyme system-Pgp Anticipated effect on FK, Csa or RAPA Suggested dose adjustm Azole Antifungals Voriconazole Inhibitor of CYP2C9, 2C19 and 3A4 Inhibition of metabolism will result in decreased clearance and increased levels. Monitor and decrease dose accordingly ↓ Csa by 50%, ↓ FK by Contraindicated with R Doses ≥ 200mg daily m CNI dose of up to 50% ↓ CNI dose by at least 5 ↓ in Csa dose of 50-75% essary May decrease FK levels Csa increases AUC of caspofungin by 35% none Monitor FK levels and justments accordingly. w/Csa ↓ caspofungin do daily and monitor LFT’ none Inhibition of metabolism will result in decreased clearance and increased levels. Monitor and decrease dose accordingly Dose dependent inhibiti effect may be more pro Csa than FK. Verapamil slightly less diltiazem Inhibition of metabolism will result in decreased clearance and increased levels. Monitor and decrease dose accordingly Induction of metabolism will result in increased clearance and decreased levels. Monitor and increase dose accordingly ↓ Csa dose by 35-50% ↓ in Csa dose of approx may be needed Inhibitor of CYP3A4 and 2D6 & pgp Inhibitor of CYP3A4 and P-gp Inhibition of metabolism will result in decreased clearance and increased levels. Monitor and decrease dose accordingly Common dosing in a PI men: Csa 10-25 mg BID, FK RAPA 0.5-2 mg qWk Anticonvulsants Phenytoin Carbamazepine Phenobarbitol Inducer of CYP3A4 Inducer of CYP3A4 Inducer of CYP3A4 Induction of metabolism will result in increased clearance and decreased levels. Monitor and increase dose accordingly Specific recommendatio ble. Monitor levels and accordingly Others Grapefruit juice Inhibitor of CYP3A4 and P -gp Inhibition of metabolism will result in decreased clearance and increased levels Induction of metabolism will result in increased clearance and decreased levels Discourage use of grape fruit/grapefruit juice po interaction is highly var cult to predict dose adju Discourage use of St. Jo Csa levels of 30-64% h Fluconazole Inhibitor of CYP2C9 and 3A4 Itraconazole Ketoconazole Inhibitor of CYP2C9 and 3A4 Inhibitor of CYP2C9, 2C19 and 3A4 Echinocandin antifungals Caspofungin none Micafungin none Calcium Channel Blockers Diltiazem Inhibitor of CYP3A Verapamil Antibiotics Erythromycin Clarithromycin Rifampin Protease Inhibitors Ritonavir Saqunavir St John’s Wort Inhibitor of CYP3A Inhibitor of CYP3A and 1A2 Inhibitor of CYP3A4 and 3A5 Inducer of CYP3A4 and P-gp Inducer of CYP3A4 and P-gp ↑ baseline Csa dose of 2 dose may be necessary Herbal Product Artagalus root (Milk Vetch Root, Huang-qi) Alfalfa (Medicago Sativa)* Bovine colostrum* Echinacea (Coneflower, Rudbeckia, Sampson Root) Ginseng (Panax ginseng) Licorice (Glycyrrhiza glabra) Vitamin E* Potential Effect Used to boost immune system, fight infection and treat heart, kidney and liver failure Appetite stimulant Used to boost immune system, build muscle and speed healing of injuries Used to boost the immune system and fight off infection Increases abstract thinking, reaction time and may be used to fight off viral infections Used to soothe respiratory infections and treat stomach ulcers Improved wound healing Zinc Used commonly to treat symptoms of the common cold St. John’s Wort ** (Hypericum perforatum) Used as a treatment for depression and sleep disorders Grapefruit juice** Common source of vitamin C Therapeutic consequence Stimulates the immune system through increased production of antibodies and T-cells. Stimulation of the immune system may result in an increased risk for rejection. Causes activation of T-cells potentially resulting in immunostimulation. May enhance immune system resulting in increased risk for rejection. Bovine colostrum has been know to contain immunostimulatory products including actoferrin and colostrinin or proline-rich polypeptide (PRP). The immunostimulatory effects may trigger a rejection episode. Should not be used in transplant recipients due to its immunostimulatory effects. Chronic use may precipitate a rejection episode. Modulation of peripheral blood mononuclear cells (PBMC) resulting in increased IL-12 production. Immunostimulatory effects may precipitate a rejection episode The glycyrrhizin in licorice has been shown to boost the immune systems T-cell count and stimulate production of interferons. Increased t-cell activation may precipitate a rejection episode. High doses of vitamin E may have immunostimulatory effects as evidenced by enhanced in vivo indexes of T cell-mediated function. Patients with underlying autoimmune disorders may be particularly susceptible to the immunostimulatory effects of vitamin E. Zinc has been shown to be able to induce cytokines, including interleukin (IL)-1, IL-6 and tumor necrosis factor (TNF)-alpha. Excessive consumption of zinc may have immunostimulatory effects. Induces activity of both CYP3A4 and P-gp resulting in decreased levels of cyclosporine, tacrolimus and sirolimus. Numerous case reports published on increased rejection rates in transplant patients taking St. Johns Wort3 Inhibition of both CYP3A4 and P-gp causes accumulation of cyclosporine, tacrolimus and sirolimus resulting in drug toxicity or over immunosuppression b. HIV in Transplantation Guidelines for the management of Immunosuppression in HIV+ transplant recipients on HAART Significant drug interactions exist with the combinations or calcineurin inhibitors and protease inhibitors. The section below provide some guidelines as to have to manage the various combinations that may be seen in a HIV+ transplant recipient IMMUNOSUPPRESSANTS Cyclosporine: CsA dose ranges for patients on NNRTIs remain at a normal-high range for non-HIV transplant patients (125-325 mg bid), with efavirenz requiring about twice the dose given with nevirapine. The doses for those patients on PIs or PIs+NNRTIs were much lower, in the range of 25 to 50 mg bid. Adding an NNRTI to a PI containing regimen generally increased the dose requirements for CsA. The table below illustrates the significant difference in Csa requirements seen with various antiretrovirals. Table courtesy of HIV-TR trial. 35 0 30 Wk 2 0 25 CsA0 20 mg/ 0 15 dose 0 10 05 00 N VP EF V 1 PI Wk 12 2 PI Wk 28 Kale etra tra Yr 1 NNR +P TI I Yr 2 NNRkale TI + tra Kaletra Kaletra Tacrolimus: Subjects on NNRTIs generally receive 1.5-2 mg bid of tacrolimus, While subjects on nnrtis + pis + tacrolimus average 0.5-1 mg bid Subjects on PIs + tacrolimus average 0.5 mg twice a week. Sirolimus: Subjects on sirolimus who are on PIs have an average dose of sirolimus around ~ 1mg once or twice a week. Subjects on NNRTIs and sirolimus average dose is about 3 mg daily. As with CsA and tacrolimus, adding a PI to an NNRTI regimen reduces the dose required and increases the dosing interval. Two subjects have also been on an NNRTI, a PI and CsA, at a lower sirolimus dose of ~ 1 mg q week. NNRTIs: Nevirapine: Nevirapine pharmacokinetics are essentially unchanged by the addition of immunosuppressants. Adding a PI to nevirapine tended to lower nevirapine levels over time by as much as 30%. Efavirenz: Pretransplant, efavirenz AUCs were high in both liver and kidney patients pretransplant. After transplantation, efavirenz AUCs tended to decrease over time Adding a PI to an efavirenz level tended to maintain efavirenz levels at intermediate levels compared with AUCs pretransplant or efavirenz alone. PIs: Common regimens: Nelfinavir and CsA (n=5): Both nelfinavir and M8 increased post transplant for up to 12 weeks, trended down for up to one year, and were elevated again at Year 2. M8:NFV AUC ratios remained low at all timepoints, usually < 0.1. PI with a ritonavir booster or on two PIs: Adding a second PI requires increases both ARV and IS levels and requires a decrease in immunosuppressant dose or increase in dosing interval or both. Two PIS with an NNRTI: Adding NVP does not affect PI levels but requires an increase in immunosuppressant dose (especially CsA). Adding EFV requires increasing the PI doses and the Immunosuppressant doses (especially CsA) Dose adjustment of Antiretrovirals Posttransplant Antiretroviral combinations that contain medications that are primarily renally cleared will need dose adjustment after a kidney transplant. It is essential that these medications are adjusted to the appropriate dose corresponding to the patients renal function posttransplant to maintain efficacy of the antiretroviral regimen. In kidney that display immediate graft function doses should be increased to full dose when the serum creatinine falls below 2.0 mg/dL In cases of delayed graft function keep the respective antiretroviral at the renal adjusted dose until the creatinine falls below 2.0 mg/dL. Below is a list of common antiretrovirals and the appropriate dose adjustment for varying degrees of renal function. The bolded medications are the ones that need dose adjustments. Entry Inhibitors Enfuvirtide (T-20, Fuzeon) Protease Inhibitors (PI’s) Amprenavir (APV, Agenerase) Atazanavir (ATV, Reyataz) Fosamprenavir (908, Lexiva, Telzir) Indinavir (IDV, Crixivan) Lopinavir/Ritonavir (LPV/RTV, Kaletra) Nelfinavir (NFV, Viracept) Nucleoside/Nucleotide Analog Reverse Transcriptase Inhibitors (NRTI’s) Abacavir (ABC, Ziagen) Combivir (AZT/3TC, Zidovudine/Lamivudine) Didanosine (ddI, Videx) Emtricitabine (FTC, Emtriva) Epzicom (ABC/3TC, Abacavir/Lamivudine) Lamivudine (3TC, Epivir) Stavudine (d4T, Zerit) Tenofovir (TDF, Viread) Trizivir (AZT/3TC/ABC, Zidovu- Ritonavir (RTV, Norvir) Saquinavir (SQV, Fortovase, Invirase) dine/Lamivudine/Abacavir) Truvada (TDF/FTC, Tenofovir/Emtricitabine) Zalcitabine (ddC, Hivid) Zidovudine (AZT, Retrovir) Non-Nucleoside Reverse Transcriptase Inhibitors (NNRTI’s) Delavirdine (DLV, Rescriptor) Efavirenz (EFV, Sustiva, Stocrin) Nevirapine (NVP, Viramune) Anti-retroviral Dose Adjustment for Hepatic and Renal Insufficiency Zidovudine Didanosine Creatinine Clearance > 60 600 mg Daily divided BID or TID Hemo or peritoneal dialysis 100 mg q6-8 hours Consider dosage reduction in renal and hepatic insufficiencies. Tablets/Powders Creatinine Clearance > 60 > 60 kg 200mg BID < 60 kg 150mg BID Creatinine Clearance 30-59 > 60 kg 200mg QD < 60 kg 150mg QD Creatinine Clearance 10-29 > 60 kg 150 mg QD < 60 kg 100 mg QD Creatinine Clearance <10 (dialysis range) > 60 kg 100 mg QD < 60 kg 75 mg QD Enteric Coated Creatinine Clearance > 60 > 60 kg 400mg QD < 60 kg 250mg QD Creatinine Clearance 30-59 > 60 kg 200mg QD < 60 kg 125mg QD Creatinine Clearance 10-29 > 60 kg 125 mg QD < 60 kg 125 mg QD Creatinine Clearance <10 (dialysis range) > 60 kg 125 mg QD < 60 kg not suitable Creatinine Clearance > 40 0.75 mg q8h Creatinine Clearance 10-39 0.75 mg q12h Creatinine Clearance < 10 0.75 mg q24h Creatinine Clearance > 50 > 60 kg 40 mg q12h < 60 kg 30 mg q12h Creatinine Clearance 26-49 > 60 kg 20 mg q12h < 60 kg 15 mg q12h Creatinine Clearance 10-25 > 60 kg 20 mg q24h < 60 kg 15 mg q24h Creatinine Clearance > 50 150 mg bid Creatinine Clearance 30-49 150 mg qd Zalcitabine Stavudine Lamivudine Creatinine Clearance 15-29 150 mg x 1 then 100 mg qd Creatinine Clearance 5-14 150 mg x1 then 50 mg qd Creatinine Clearance < 5 50 mg x1 then 25 mg qd Should not be prescribed if Creatinine Clearance < 50 or patient < 50kg Combivir Delavirdine, Saquinavir, Principally metabolized by the liver, therefore caution is recommended in hepatic insufficiency but specific data are not available to guide dose Ritonavir, Nelfinavir adjsutments Hepatic insufficiency 600 mg q8h Indinavir Child-Pugh 5-8 450 mg bid, Amprenavir Child-Pugh 8-12 300 mg bid Creatinine Clearance 30 – 49 300 mg every 48 hours Tenofovir Creatinine Clearance 10 – 29 300 mg every 72 – 96 hour Creatinine Clearance <10 300 mg every 7 days Hemodialysis 300 mg every 7 days following hd Creatinine Clearance >50 200 mg QD Emtricitabine Creatinine Clearance 30-49 200 mg Q 48h Creatinine Clearance 15-29 200 mg Q 72h Creatinine Clearance <15 200 mg Q 96h c. Dose adjustment in renal failure Drug name Acyclovir IV (Zorivax) CrCl > 50 ml/min 25-50 ml/min 10-25 ml/min <10 ml/min Hemodialysis Dose 5-10 mg/kg 5-10 mg/kg 5-10 mg/kg 2.5-5 mg/kg 2.5-5 mg/kg Ampicillin/ Sulbactan (Unasyn) > 30 ml/min 15-29 ml/min <15 ml/min Hemodialysis 1.5-3 gm 1.5-3 gm 1.5-3 gm 1.5-3 gm Amoxacillin/ Sulbactam (Augmentin) > 30 ml/min 10-30 ml/min <10 ml/min 250-500 mg 250-500 mg 250-500 mg Aztreonam (Azactam) > 30 ml/min 10-30 ml/min <10 ml/min 500mg-2 gm 500mg-1 gm 125-500 mg Interval q 8 hrs q12hrs q24hrs q24hrs q24hrs post HD q 6-8 hrs q12hrs q24hrs q24hrs post HD q 8 hrs q12 hrs q24hrs post HD q8-12hrs q8-12hrs q8-12hrs Cefepime (Maxipime) > 60 ml/min 30-60 ml/min 10-29 ml/min <10 ml/min 1-2 gm 1-2 gm 500mg-1gm 250-500mg q12h q24h q24h q24h Comments Maintain adequate hydration Infuse over at least one hour to avoid nephrotoxicity Max daily dose: 3 gm q 6hrs. Dose q 8h in elderly w/normal renal function Alternate dosing w/normal renal function 875 mg q 12h Decrease dose by 25% in pts w hepatic insufficiency, especially if concurrent renal impairment Ciprofloxacin (Cipro) PO > 30 ml/min 10-30 ml/min <10 ml/min 250-750 mg 250-750 mg 250-750 mg Enoxaparin (Lovenox) DVT prophylaxis Enoxaparin (Lovenox) DVT/PE treatment > 30 ml/min <30 ml/min 30 mg 30 mg q12h q24h q24 post HD q12h q24h > 60 ml/min 30-60 ml/min <30 ml/min 1 mg/kg 0.84 mg/kg 1 mg/kg q12h q12h q24h Fluconazole (Diflucan) >50 ml/min 11-50 ml/min <10 ml/min 100-400 mg 100-200 mg 100-200 mg q24h q24h q48h Do not dose adjust for transient increases in Scr due to FK toxicity Ganciclovir (Cytovene) Induction >70 ml/min 50-69 ml/min 25-49 ml/min 10-24 ml/min <10 ml/min >70 ml/min 50-69 ml/min 25-49 ml/min 10-24 ml/min <10 ml/min >70 ml/min 30-69 ml/min 20-29 ml/min 5-20 ml/min Hemodialysis 5 mg/kg 2.5 mg/kg 1.25 mg/kg 0.625 mg/kg 0.625 mg/kg 5 mg/kg 2.5 mg/kg 1.25 mg/kg 0.625 mg/kg 0.625 mg/kg 500 mg 500 mg 500 mg 250 mg 250 mg Induction dose in used for 2 wks post OLT or when there is active CMV infection >50 ml/min 20-50 ml/min 10-20 ml/min Hemodialysis >50 ml/min 25-50 ml/min 10-25 ml/min <10 ml/min >40 ml/min 20-40 ml/min <20 ml/min Hemodialysis >30 ml/min 15-30 ml/min 500 mg 250 mg 250 mg 250 mg 1 gm 1 gm 500 mg 500 mg 4.5 gm 2.25 gm 2.25 gm 2.25 gm 15-20 mg/kg/d 15-20 mg/kg/d then 7-10 mg/kg/d 7-10 mg/kg/d 10 mg/kg 8-12 mg/kg/d 8-12 mg/kg/d q12h q12h q24h q24h qHD q24h q24h q24h q24h qHD q6h q6-8hrs q8-12hrs q12h q12h post HD q24h q24h q48h q48h q8h q12h q12h q24h q6-8h q6h q8h q12h q6-8h q6-8h x 2 d q12h q12-24h post HD q6-8h q6-8h x 2 d Ganciclovir (Cytovene) maintenance Imipenem (Primaxin) Levofloxacin (Levaquin) Meropenem (Merrem) Piperacillin/ Tazobactam (Zosyn) Trimethoprim/ Sulfamethoxazole (Bactrim) IV or PO PCP treatment <15 ml/min Hemodialysis Trimethoprim/ >30 ml/min Sulfamethoxazole 15-30 ml/min Separate administration of Ca, Mg, Zn, Fe, Al by 2 hrs Induction dose in used for 2 wks post OLT or when there is active CMV infection CVVHD- Dose for CrCl of 20-40 ml/min Dose is in mg of TMP/kg/day Dose is in mg of TMP/kg/day (Bactrim) Other indications Valganciclovir (Valcyte) Induction Valganciclovir (Valcyte) Maintenance/ prophylaxis <15 ml/min Hemodialysis >60 ml/min 40-59 ml/min 25-39 ml/min 10-24 ml/min <10 ml/min >60 ml/min 40-59 ml/min 25-39 ml/min 10-24 ml/min <10 ml/min then 4-6 mg/kg/d 4-6 mg/kg/d 6 mg/kg 900 mg 450 mg 450 mg 450 mg Use ganciclovir 900 mg 450 mg 450 mg 450 mg Use ganciclovir q12h q12-24h post HD BID BID QD QOD QD QD QOD 2x/week