Syllabus
advertisement

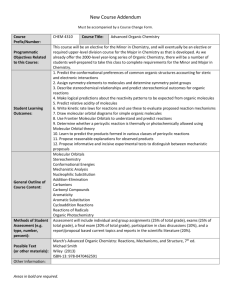
http://web.uvic.ca/~chem213/chem213.htm Instructor: Dr. Devin Mitchell Office: Ell 327 Email: devinpm@uvic.ca CHEMISTRY213 Course Outline - SUMMER 2009 PRE- OR CO-REQUISITE IS CHEM 231 - NO EXCEPTIONS 1. Textbooks YOU WILL NEED THE COURSE MANUAL : PRACTICAL SPECTROSCOPY Chem 213 Manual - FALL 2008/2009 available in the bookstore. This contains a synopsis of all lectures, copies of all class overheads, problems, assignments, mid-terms and finals, as well as tutorial information. NO OTHER TEXT IS NECESSARY, however there are two small cheap texts (i) “Foundations of Spectroscopy” by Simon Duckett and Bruce Gilbert (Oxford Chemistry Primers - ISBN 0198503350, and (ii) “Inorganic Spectroscopic Methods” by Alan K Brisdon (Oxford Chemistry Primer ISBN 0198559496) which may be useful. The organic aspects of the course are also reasonably well covered in most general organic chemistry texts,which most of you will have already purchased for the pre- or corequisite, Chem 231, e.g. “Organic Chemistry”, J. McMurry, 6th Edn, Chapters 12-14 or previous texts, e.g. "Organic Chemistry", S. Ege, 4th Edn, Chapters 10, 11, or “Organic Chemistry”, T.W.G. Solomons, Chapter 14 Students who are continuing in chemistry, especially organic chemistry, may wish to purchase one of the following specialist spectroscopy texts (check prices of each!). "Introduction to Spectroscopy", D.L. Pavia, G.M. Lampman, and G.S. Kriz. 3rd Edn 2001 ISBN 003-031961-7 strongly recommended for Chem Majors and Honors students Other problems can be found in: "Spectroscopic Methods in Organic Chemistry", Dudley H. Williams, and Ian Fleming. "Organic Spectroscopy", William Kemp. “Introduction to Organic Spectroscopy”, J.B. Lambert, H.F. Shurvell, D.A. Lightner, R.G. Cooks. The following texts are on reserve in the Library: Bak, B.N., Elementary Introduction to Molecular Spectra Banwell, C.N., Fundamentals of Molecular Spectroscopy Barrow, G.M., Introduction to Molecular Spectroscopy Pavia, D.L., Introduction to Spectroscopy Lambert, J.B., Shurwell, H.F., Lightner, D. and Cooks, R.G., Introduction to Organic Spectroscopy 2. Grading The final grade for the course will be made up as follows: Assignments/Quizzes 0-6% (optional) Mid-term (50 minutes) 20% Final exam (3 hours) 49-55% Tutorial Lab 25% There will be six assignments during the term. These will not be handed in and marked but there will be a short, open book, i.e. bring in your answers to the assignments (photocopied material is prohibited), multiple choice quiz on each assignment. If you have completed the assignment properly then it should be possible to score 100% on the quiz. If you do not write any quiz (by choice or for a medical reason) then the percentage allotted to your final exam will be increased accordingly. The assignments form only a small percentage of your final mark because they are designed mainly as study aids, not tests. The variable percentage allocated to quizzes means that any individual quiz mark which is less than the final exam mark will be discarded. Thus, there is NO DISADVANTAGE TO WRITING A QUIZ, even if you perform poorly. In order to pass Chem 213, students must obtain a passing grade in both the lecture and the tutorial-lab portions of the course. Students who perform poorly (40-49%) on the final, but pass the course on aggregate, may only receive a D grade. A score of <40% on the final will normally result in an F grade for the course, regardless of aggregate score. Credit will NOT be given for both this course and 233 or 314 or 316. 3. Syllabus A) General (1 week) Electromagnetic spectrum, interaction with matter, Boltzmann distribution, spectrometer design. B) Infrared - organic applications (1 week) Correlation charts, analysis of spectra, survey of main functional groups. C) Infrared - theory and inorganic (1-2 weeks) i) diatomic molecules, simple harmonic oscillator, selection rules, fundamentals, overtones, examples. Microwave spectroscopy, rotating/vibrating diatomic, CO spectrum. ii) polyatomic molecules, 3N-6(5) vibrations, H2O, CO2 iii) structure determination: complete analysis, finger-printing, group frequencies. Typical AB3, AB4, AB5 and AB6 examples. D) NMR - introduction (1 week) Nucleus as a magnet, effect of external fields, chemical shifts, coupling constants. E) NMR - organic applications (3 weeks) Chemical shifts - electronegativity, hybridisation, aromaticity. Coupling constants - alkenes, geometry, protons on O, N, non-first order AB, brief AB2 etc. 13C spectra -abundance, decoupling, shifts. Structure assignment. F) NMR - theory and multinuclear (2 weeks) Nuclear structure and spin, effect of B0, spectrometer design. Chemical equivalence, integration, chemical shift. Magnetic equivalence, coupling mechanism and constant, 1st order patterns. Examples from 1H, 11B, 13C, 19F, 119Sn, 195Pt, etc. G) Mass spectroscopy (1 week) Principles and spectrometer design, determination of molecular formula (isotope ratios, exact mass). Fragmentation - guide to groups present. H) UV/Visible (1 week) Molecular energy levels and transitions, →*, n→* etc. Energies and intensities. Spectrometer design. Types of chromophore and shifts, conjugation, conformation and geometry, substituent effects in aromatics. Applications to transition metal complexes (brief). IMPORTANT DATES: MID-TERM EXAM JUNE 18th QUIZ DATES - ALL THURSDAYS QUIZ 1 MAY 14 QUIZ 2 MAY 28 QUIZ 3 JUNE 4 QUIZ 4 JUNE 11 MIDTERM JUNE 18 QUIZ 5 JUNE 25 QUIZ 6 JULY 16