Review Sheet for Scientific Method and Elements Quiz
advertisement

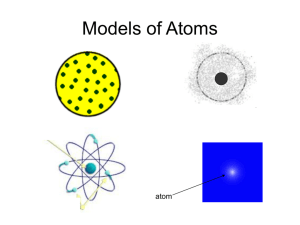
Review Sheet for Scientific Method and Elements Quiz 1. Name the steps of the scientific method. 2. What is the scientific method? 3. What form does the problem always have to be in? 4. Give an example of a problem. 5. Why do you gather information before you write your hypothesis and do the experiment? 6. What are some advantages and disadvantages of the following sources? Books, internet, expert 7. What is a hypothesis? 8. Give an example of a hypothesis in correct form. 9. Why is it important to repeat experiments? 10. How many variables can you have in an experiment? Explain why, using examples. 11. Why is it important to have a control? Define control. 12. What’s the difference between recording observations and analyzing observations? 13. What should you include in your conclusion? 14. What does refute mean? 15. Can you ever say that your hypothesis was right or wrong? Why? 16. What are the three particles found in an atom? 17. What is the nucleus? 18. What kind of charge does a proton have? Electron? Neutron? 19. What kinds of particles are found in the nucleus? 20. What is the reason that an atom is balanced even though it has protons that are positive and electrons that are negative? 21. Where are the electrons found in an atom? 22. What does the present modern model of an atom look like? 23. Where do electrons with lower energy move? What about the electrons with higher energy? 24. Out of the three particles, which particles have a significant mass? 25. How many electrons equal the mass of one proton? 26. What unit is used to describe the mass of atomic particles? 27. What does atomic number describe? 28. Are there any elements that have the same atomic number? Explain. 29. What is an isotope? 30. What is a mass number? 31. What is atomic mass? 32. What is the difference between Nitrogen-15 and Nitrogen-14? 33. How come scientists aren’t sure if their models of atoms are correct? 34. What is the “chemists’ calendar” called? 35. Who published the first periodic table of elements? 36. What is included in each element box? 37. What are the abbreviations for elements called? 38. Why are some abbreviations different letters than their “regular” full name? 39. Why does the atomic mass of an element have a decimal? 40. What are the horizontal rows in a periodic table called? What about the vertical columns? 41. How do you know what family an element is in? 42. As you go from left to right in a period, what happens to the reactivity of elements? 43. Which group reacts violently with water? Which group reacts violently with Group 1? Which group does not react at all? 44. How can you tell if an element is a metal, metalloid, or non-metal? How can you tell if an element is a solid, liquid, or gas? How can you tell if an element is not found in nature? 45. Tell how many protons, electrons, and neutrons are in each of the elements in Group 1.