Crystal Structure and Functional Analysis of Glyceraldehyde
advertisement

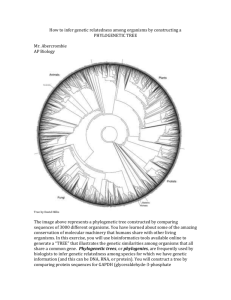
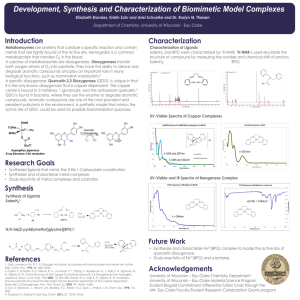
Crystal Structure and Functional Analysis of Glyceraldehyde-3-phosphate Dehydrogenase from Oryza Sativa Yueh-Chu Tien (田岳衢)1,2, Yi-Hung Lin (林易弘)2, Shou-Lin Chang (張壽麟)1, and Chun-Jung Chen (陳俊榮)2 1 Department of Life Science, National Tsing Hua University, Hsinchu, Taiwan 2 National Synchrotron Radiation Research Center, Hsinchu, Taiwan Cytoplasmic Oryza sativa GAPDH (OsGAPDH)is an enzyme belong to GAPDH family (E.C. 1.2.1.12) and essential in glycolysis pathway. This enzyme catalyzes phosphorylation of glyceraldhyde-3-phosphate (G3P) to 1,3-biphosphoglycerate (BPG) only using the cofator NAD+ to accept electrons from substrates. Another kind GAPDH belong to subfamily (E.C. 1.2.1.13) which could use both NAD+ and NADP+ as cofactor transferring BPG to G3P. The OsGAPDH protein structure is determeined by X-ray diffraction method. Three crystallizational conditons perform three structures: NAD-free, NAD-bound and sulfate-soaked. Similar to the published GAPDH structure, OsGAPDH shows homotetramer form and each subunit could be seperated to three domains: NAD-binding domain, catalytic domain and S-loop domain. NAD+ bind to OsGAPDH by hydrogen bonds directly and intermediated by water. Some residues form positive grooves to attract sulfate molecules which are used to simulate phosphate groups of BPG. Some other features found in these structures have not been reproted before. Phe37 is found forming a bottleneck which may improve NAD+ binding, but this assumption should be proved advancely. Phe37 is also involved in NAD-specific feature, together with Pro193 and Asp35 which have been reported as key residues in NAD-specific feature.Align amino acid sequence with other organisms, reveal that Phe37 only exists in higher organism, but lacks in lower organisms. In contrast, Pro193 and Asp35 exist in each GAPDH belong to E.C.1.2.1.12. This phenomenon may be caused by evalution and results in different biochemical performance between different organisms.