Alkene Discussion Problems
advertisement

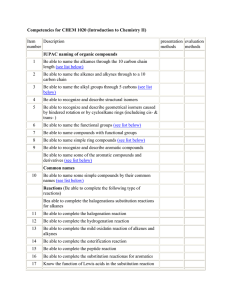
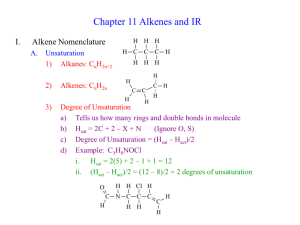
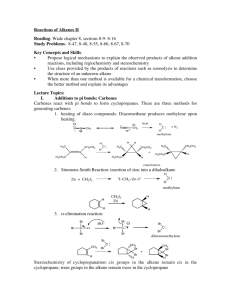
Name __________________________ Period______ Advanced Chemistry Discussion Problems: C13 – Alkenes, Alkynes, and Aromatic Compounds! 13.1: Alkenes and Alkynes! 1. Label the following organic compounds as saturated or unsaturated. a. Alkanes b. Alkenes c. Alkynes 2. What process is used to make vast quantities of alkenes? What is a simple alkene that is produced in the largest quantity than any other organic molecule? 13.2: Naming Alkenes and Alkynes! 1. Draw the condensed structure of the following: a. 1,4-Hexadiene b. Cyclopentene c. Acetylene d. 5-Methyl-1-heptene e. 1,6-Octadiene f. 2,5-Octadiene g. 2,4-Octadiene h. 3-Methyl-1-butene i. 3-Ethyl-2,2-dimethyl-3-hexene j. 4,4-Dimethyl-2-pentyne 2. What is the IUPAC name of the following: a. CH2=CHCH=CH2 b. CH3CH2CH2C=CCH3 | | H3C CH3 c. (CH3CH2)2CHCCCH3 d. (CH3)2C=CHCH3 13.3: Cis-Trans Isomerism! 1. What is the cause of cis-trans isomerism? When does it occur? 2. What is the IUPAC name of the following compounds: H CH3 | | a. CH3CHCH2CHC=C | | | CH3 CH3 H H3C C2H5 | | b. C=C | | H Cl H C2H5 | | c. C=C | | H3C Cl H CHCl-CH3 | | d. C=C | | H CH3 3. Which of the following exist as cis-trans isomers? Draw both isomers. a. 3-Heptene b. 2-Methyl-2-hexene c. 5-Methyl-2-hexene d. 3,4-Dimethyl-3-hexene 13.4: Properties of Alkenees and Alkynes! 1. Compare and contrast the properties of alkenes to alkanes. 2. What properties do alkenes and simple aromatics have in common? 13.5: Organic Reactions! 1. Summarize, compare, and contrast addition, elimination, substitution, and rearrangement reactions. 2. Chemical reactions involving double bonds are generally what type of organic reaction? 3. Bromination, chlorination, hydration, and hydrogenation are all examples of what type of organic reaction? 4. Classify the following reactions as an addition, elimination, substitution, or rearrangement: a. H2C=CH2 + HCl -> CH3CH2Cl b. CH3Br + NaOH -> CH3OH + NaBr 13.5-7: Reactions!!! 1. When an alkene undergoes hydrogenation, the product is what type of organic molecule? 2. When 2-butene reacts completely with bromine, what is the product? 3. What reactant should be used to convert propane to 2-chloropropane? 4. Which reactant should be used to convert propane to 1,2-dichloropropane? 5. According to Markovnikov’s rule, when HCl reacts with the molecule shown, which product will result? (CH3)2C+CHCH3 + HCl -> ??? 6. When an alkene undergoes a hydration reaction, what is the product? 7. What commonly used mechanism is the accepted explanation for alkene reactions? 13.8: Alkene Polymers! 1. What is the monomer unit used to produce polypropylene? 2. What is the starting material for polymerization reactions? 3. Name the polymer formed from CH2=CH2. 13.9: Aromatic Compounds and the Structure of Benzene! 1. Describe the structure common to all aromatic compounds. 2. Describe how resonance explains the properties of aromatic compounds based on a structure that is an average among two possibilities. 3. Draw benzene and toluene. 13.10: Aromatic Nomenclature 1. When the aromatic ring is named as a substituent, functional group, or side chain, it is referred to as the ___________ group! 2. Draw the following: a. para-dimethylbenzene b. phenol c. benzoic acid d. a phenyl group e. m-Isopropylphenol f. o-Dichlorobenzene 3. Name the following: a. b. c. 13.11: Reactions of Aromatic Compounds! 1. What is the most common reaction involving aromatics? 2. List some common reactions for benzene!