Linaclotide shared care guidelines
advertisement

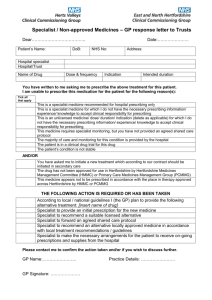
Shared Care Protocol –remains open to review in light of any new evidence Amber = To be initiated and titrated to a stable dose in secondary care with follow up prescribing and monitoring by primary care. Linaclotide (Constella®) Shared Care Guideline for the use of Linaclotide in moderate to severe Irritable Bowel Syndrome with constipation unresponsive to conventional laxatives. Introduction Indication/Licensing information Linaclotide is indicated for the symptomatic treatment of moderate to severe irritable bowel syndrome with constipation (IBS-C) in adults. Irritable Bowel Syndrome with Constipation (IBS-C) diagnosed Place in therapy: Give Information Dietary Management First line Laxatives Physical Exercise Second line Laxatives Complementary and Alternative Medicines may be considered, although no specific treatments have been assessed or endorsed by the Area Prescribing Committee. Combination Laxatives Consider trial of Linaclotide. Pharmacology Linaclotide is a Guanylate Cyclase-C receptor agonist (GCCA) with visceral analgesic and secretory activities. Both linaclotide and its active metabolite bind to the GC-C receptor on the luminal surface of the intestinal epithelium, leading to an increase in concentrations of cyclic guanosine monophosphate (cGMP), both extracellularly and intracellularly. Extracellular cGMP decreases pain-fiber activity, resulting in reduced visceral pain in animal models. Intracellular cGMP causes secretion of chloride and bicarbonate into the intestinal lumen, through activation of the cystic fibrosis transmembrane conductance regulator (CFTR), which results in increased intestinal fluid and accelerated transit. Dosage and administration The recommended dose is one capsule (290 micrograms) once daily, at least 30 minutes before a meal. Physicians should periodically assess the need for continued treatment. The efficacy of linaclotide has been established in double-blind placebo-controlled studies for up to 6 months. If patients have not experienced improvement in their symptoms after 4 weeks of treatment, the patient should be re-examined and the benefit and risks of continuing treatment reconsidered. Constella® is available as hard capsules containing 290micrograms of linaclotide per capsule. Linaclotide Shared care Guideline Date Prepared: 30th October 2014 Review Date: 30th October 2016 Page 1 of 6 Shared Care Protocol –remains open to review in light of any new evidence Amber = To be initiated and titrated to a stable dose in secondary care with follow up prescribing and monitoring by primary care. Responsibilities of the specialist initiating treatment Summary To assess the suitability of the patient for treatment. To discuss the benefits and side effects of treatment with the patient/carer and the need for long term monitoring if applicable. To perform baseline tests and if appropriate routine tests until the patient is stable. To prescribe for the first 12 weeks of treatment To ask the GP whether they are willing to participate in shared care. To provide the GP with a summary of information relating to the individual patient to support the GP in undertaking shared care (See Shared care request form in Appendix A). To advise the GP of any dosage adjustments required, monitoring required, when to refer back, and when and how to stop treatment (if appropriate). To advise the GP when the patient will next be reviewed by the specialist. To monitor the patient for adverse events and report to the GP and where appropriate Commission on Human Medicines/MHRA (Yellow card scheme). To provide the GP with contact details in case of queries. Baseline Tests Baseline U&Es will be completed prior to starting treatment with linaclotide. Routine Tests There is no routine monitoring necessary with the administration of linaclotide. However, it is advised that U&E’s are checked periodically in patients predisposed to electrolyte disturbances, should prolonged or severe diarrhoea occur, or as clinical judgement dictates. Disease monitoring Patients will be reviewed by the Specialist team 8 weeks after initiation of treatment with linaclotide, to assess efficacy and tolerability. If the patient is gaining significant benefit, a further 4 weeks of treatment should be supplied, and the GP approached to consider shared care. Continued efficacy and tolerability should be reviewed every 3 months. Responsibilities of other prescribers Acceptance of Responsibility by the Primary Care Clinician It is optional for GPs to participate in taking on responsibility for shared care for the patient. GPs will take on shared care only if they are willing and able. Summary To reply to the request for shared care as soon as possible. To prescribe and adjust the dose as recommended by the specialist. To ensure there are no interactions with any other medications initiated in primary care. To continue monitoring as agreed with secondary care To refer back to the specialist where appropriate. For example: o Patient or general practitioner is not comfortable to continue with the existing regime due to either change in condition or drug side effects. o Advice in respect of concordance. o Special situations, (e.g. Pregnancy) Discontinue the drug as directed by the specialist if required To identify adverse events if the patient presents with any signs and liaise with the hospital specialist where necessary. To report adverse events to the specialist and where appropriate the Commission on Human Medicines/MHRA (Yellow card scheme). Continued efficacy and tolerability should be reviewed every 3 months. Linaclotide Shared care Guideline Date Prepared: 30th October 2014 Review Date: 30th October 2016 Page 2 of 6 Shared Care Protocol –remains open to review in light of any new evidence Amber = To be initiated and titrated to a stable dose in secondary care with follow up prescribing and monitoring by primary care. Clinical Particulars BNF therapeutic class Cautions and Contraindications 1.6.7 Other drugs used in Constipation (Gastrointestinal System) Linaclotide is contraindicated in patients with gastrointestinal obstruction. Use in patients with inflammatory bowel disease is not recommended due to lack of safety and efficacy data in this patient group. It should be used with caution in patients predisposed to fluid and electrolyte disturbances (e.g. elderly, patients with cardiovascular disease, diabetes, hypertension). No dosage adjustments are required for elderly patients, or those with hepatic or renal impairment. Adverse Drug Reactions The most frequently reported adverse reaction associated with linaclotide therapy was diarrhoea, mainly mild to moderate in intensity, occurring in less than 20% of patients. In rare and more severe cases, this may – as a consequence – lead to the occurrence of dehydration, hypokalaemia, blood bicarbonate decrease, dizziness, and orthostatic hypotension. Patients should be made aware of the possible occurrence of diarrhoea during treatment. Should prolonged (e.g. more than 1 week) or severe diarrhoea occur, medical advice should be sought and temporary discontinuation of linaclotide until diarrhoea episode is resolved may be considered. Regarding duration of diarrhoea, duration of more than 28 days was reported in 21% of patients with diarrhoea; approximately one third of diarrhoea cases resolved within 7 days Other common adverse reactions (>1%) were abdominal pain, abdominal distension and flatulence. Monitoring There is no routine monitoring necessary with the administration of linaclotide. It is advised that U&E’s are checked periodically in patients predisposed to electrolyte disturbances, should prolonged or severe diarrhoea occur, or as other clinical circumstances dictate. Interactions No drug-drug interaction studies have been performed for linaclotide, although the drug is rarely detectable in plasma following administration of the recommended clinical doses. In vitro studies have shown that linaclotide is neither a substrate nor an inhibitor/inducer of the cytochrome P450 enzyme system and does not interact with a series of common efflux and uptake transporters. Taking linaclotide with or after food produced more frequent and looser stools, as well as more gastrointestinal adverse events, than when taking it under fasting conditions. Concomitant treatment with proton pump inhibitors, laxatives or NSAIDs may increase the risk of diarrhoea. In cases of severe or prolonged diarrhoea, absorption of other oral medicinal products may be affected. The efficacy of oral contraceptives may be reduced and the use of an additional contraceptive method is recommended to prevent possible failure of oral contraception. Caution should be exercised when prescribing medicinal products absorbed in the intestinal tract with a narrow therapeutic index such as levothyroxine as their efficacy may be reduced. Linaclotide Shared care Guideline Date Prepared: 30th October 2014 Review Date: 30th October 2016 Page 3 of 6 Shared Care Protocol –remains open to review in light of any new evidence Amber = To be initiated and titrated to a stable dose in secondary care with follow up prescribing and monitoring by primary care. Communication Specialist to GP The specialist will inform the GP when they have initiated linaclotide. When the patient is near completing the satisfactory initiation period, the specialist will write to the GP to request they take over prescribing and where possible give an indication as to the expected length of treatment. The Specialist will also send a Shared care request form to support the GP in undertaking shared care. (Appendix A) GP to specialist If the GP has concerns over the prescribing of linaclotide, they will contact the specialist as soon as possible. Contact names and details Contact Details Telephone number Email Dr K Kapur, Consultant Gastroenterologist, BHNFT 01226 432542 Kapil.kapur@nhs.net Dr A S Soliman, Consultant Gatsroenterologist, BHNFT 01226 432522 a.soliman@nhs.net Dr E Said, BHNFT Gastroenterologist, 01226 432910 Elmuhtady.said@nhs.net Debbie West, Lower GI Nurse Specialist, BHNFT 01226 436371 Deborah.west3@nhs.net Medicines Information Service, BHNFT 01226 432857 medicinesinformation@nhs.net Gillian Smith, Medicines Information Pharmacist, BHNFT 01226 432857 gilliansmith2@nhs.net Consultant References 1. BNF, accessed online via www.medicinescomplete.com 2. Constella Summary of Product Characteristics (SmPC) accessed online via http://www.medicines.org.uk/emc/medicine/27499 3. Rao S, Lembo AJ, Shiff SJ et al. (2012) A 12-week, randomized, controlled trial with a 4-week randomized withdrawal period to evaluate the efficacy and safety of linaclotide in irritable bowel syndrome with constipation. American Journal of Gastroenterology 107: 1714–24 4. Chey WD, Lembo AJ, Lavins BJ et al. (2012) Linaclotide for irritable bowel syndrome with constipation: a 26-week, randomized, double-blind, placebo-controlled trial to evaluate efficacy and safety. American Journal of Gastroenterology 107: 1702–12 5. NICE CG61: Irritable bowel syndrome in adults: diagnosis and management of irritable bowel syndrome in primary care. Accessed online via http://www.nice.org.uk/guidance/cg61 Development Process This guidance has been produced by Gillian Smith, Lead Pharmacist for Medicines Information and Cardiology, BHNFT, in consultation with Dr Kapil Kapur, Consultant Gastroenterologist and Clinical Director for Medicine, BHNFT, following an AMBER classification status of Linaclotide by the Barnsley Area Prescribing Committee. This guideline has been subject to consultation and endorsement by the Area Prescribing Committee on 12th November 2014 and by the LMC on 10th February 2015. Linaclotide Shared care Guideline Date Prepared: 30th October 2014 Review Date: 30th October 2016 Page 4 of 6 Shared Care Protocol –remains open to review in light of any new evidence Amber = To be initiated and titrated to a stable dose in secondary care with follow up prescribing and monitoring by primary care. Appendix A – Shared Care request form (Amber) Specialist to complete when requesting GP to enter a shared care arrangement. GP to return signed copy of form. Both parties should retain a signed copy of the form in the patient’s record. From (Specialist): To (GP): Patient details Name: ID Number: Address: DOB: Diagnosed condition: Amber Drug details Drug name: Linaclotide Date of initiation: Dose: 290mg ONCE daily Length of treatment: The patient will be reviewed by the Consultant on: The patient should be reviewed by the GP by: Monitoring The following monitoring should be undertaken by the GP: Parameter Results Frequency Baseline U&E Sodium = Potassium = Urea = Creatinine = Date next due (please specify if not routinely indicated) As clinical circumstances dictate. Consider monthly check in patients predisposed to electrolyte disturbances, according to clinical judgement. U&E Linaclotide Shared care Guideline Date Prepared: 30th October 2014 Review Date: 30th October 2016 Page 5 of 6 Shared Care Protocol –remains open to review in light of any new evidence Amber = To be initiated and titrated to a stable dose in secondary care with follow up prescribing and monitoring by primary care. Communication Consultant Telephone number: Fax number: Email address: Specialist Nurse Telephone number: Fax number: Email address: Confirmation of acceptance of shared care Specialist (Doctor/Nurse) name: Specialist (Doctor/Nurse) signature: Date: I, Dr …………………………….., can confirm I : □ accept the request to participate in shared care for the patient named above. □ reject the request to participate in shared care for the patient named above. The reason for this being ……………………………………………………………………………………….. GP signature: Linaclotide Shared care Guideline Date Prepared: 30th October 2014 Review Date: 30th October 2016 Date: Page 6 of 6