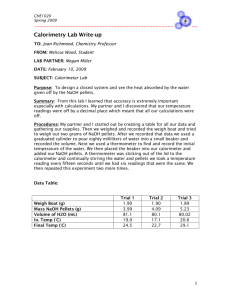
PERCENT POTASSIUM HYDROGEN
advertisement

PERCENT POTASSIUM HYDROGEN PHTHALATE IN AN UNKNOWN SAMPLE The purpose of this experiment is to determine the mass percent potassium hydrogen phthalate in an unknown sample, using neutralization titration. The reaction is KHC8H4O4 (s) + NaOH (aq) H2O (l) + Na1+ (aq) + K1+ (aq) + C8H4O42– (aq) The indicator for the endpoint is the familiar phenolphthalein indicator, which turns pink in basic solution. This experiment is a test of your titration technique, so half of your grade is determined by your accuracy and precision. You and your partner may standardize the NaOH together, but will have individual unknowns. Procedure 1. Preparation of the NaOH solution (do before coming to evening lab) CAUTION! Sodium hydroxide is extremely corrosive. You must wear goggles, and never handle the NaOH pellets with your fingers. Use tweezers to pick up any dropped pellets and dispose of them down the drain. If you get it on your skin, immediately rinse with lots and lots and lots of cold water. Prepare 500 mL of 0.1 M NaOH : calculate the required mass of NaOH and weigh it out in a small weighing cup. Don’t worry about getting the exact mass; you will determine its concentration later. Put 500 mL of deionized water into a clean 600 mL beaker and add the pellets. Stir thoroughly (use a magnetic stirrer) until the pellets are dissolved. Store your solution in a clean, labeled bottle. 2. Standardization of NaOH solution. Fill a clean dry buret with your NaOH solution (if the buret is not dry, consult the instructor). Note: never, never, NEVER pour above your head!! Take down the buret and hold it below face level. Be sure the stopcock is closed before you fill it (!). Run some NaOH solution into a waste beaker to remove air bubbles from the buret tip. Weigh some dry KHC8H4O4 (about 0.2 g) into a clean 150 mL beaker and record the exact mass. Add about 30 mL deionized water, 3-5 drops of phenolphthalein solution, and a clean stir bar. Stir constantly on the stir plate as you titrate carefully to the pale pink endpoint. The solid will dissolve as you titrate, so be sure all of it is dissolved and none is clinging to the sides of the flask (use your wash bottle to rinse it) before you accept the endpoint. Calculate the concentration of the NaOH solution from the balanced chemical equation. Rinse the beaker and repeat the titration until you are satisfied with the precision of the result (3 sigfigs). This will require at least two trials and possibly more. 3. Analysis of the unknown sample. Use the same procedure as above, using about 0.2 – 0.4 grams of unknown sample. Calculate the mass % KHC8H4O4 in the unknown, and repeat the procedure until you are satisfied with your precision. Discussion Report the % KHC8H4O4 in the unknown. Discuss any problems in the procedure and any data omitted from the final result.