ENGLISH IV Ta
advertisement

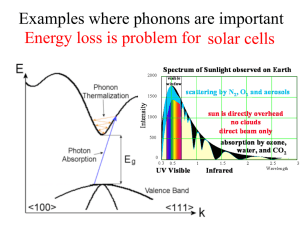
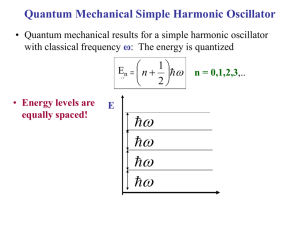
STATISTICAL MECHANICS 2002 –2003 Semester II N. Iwamoto FINAL EXAMINATION (お持ち帰り用期末試験) Thursday, 6 February – 5 pm, Thursday, 13 February 2003 1. The turbocharger of an internal combustion engine consists of a compressor and an intercooler. First, the compressor increases the air pressure by compression. The energy for compression comes from the exhaust gas, which is otherwise discarded without doing any work. During compression, which is essentially an adiabatic process, the temperature of the air also rises and the air density does not increase as much as it would in isothermal compression. In order to increase the air density, the air is then cooled by the intercooler. With these processes, compressed air with a moderate temperature is created, which is then fed into the cylinder with richer fuel in order to produce more power without increasing the displacement of the engine. Answer the following questions. (a) The air is mostly composed of two types of diatomic molecules—80 % N_2 and 20 % O_2. There are translational, rotational and vibrational degrees of freedom for diatomic molecules. The specific heat of the air at constant volume is (3/2)k_B up to about 100 C = 373 K, where k_B is the Boltzmann constant. Explain this value. Which degrees of freedom contribute to the specific heat at temperatures below 100 C? (b) Suppose that the boost pressure of the compressor is 0.46 atm, i.e., the air is compressed from 1 atm to 1.46 atm by the compressor. Let the initial temperature of the air be 30 C = 303 K. What is the temperature of the air after compression? Assume that compression is done adiabatically. (c) The intercooler cools the air by 10 K. How much heat must be extracted from the air in order to lower the temperature by 10 K? Assume that this is an isochoric process, i.e., the volume of the air is kept constant. Answer in Joule/liter. (d) Assume that the engine power is proportional to the number density of the air fed into the cylinder. What is the power of this turbo-charged engine if the normal engine (without a turbocharger) produces 230 hp? * hp: horsepower ** In 1979, Nissan first mass-produced the turbocharged engine (2,000 cc L20ET) for the domestic market (the Cedric/the Gloria), which had a boost pressure of 350 mmHg = 0.46 atm. 2. A collection of the quanta of lattice oscillations, which are called phonons, may be treated as a bose gas with zero chemical potential. This is because phonons may be freely created or absorbed. The Einstein model (A. Einstein 1907; Nobel Prize in Physics 1922) treats lattice oscillations as a collection of harmonic oscillators (which are bosons). [cf. Homework No. 5] On the other hand, the Debye model (P. Debye 1912; Nobel Prize in Chemistry 1936) treats lattice oscillations as a bose gas with a cut-off frequency (or cut-off wave number), which is associated with the degrees of freedom of lattice oscillations in a solid. [ cf. R. Kubo, Statistical Mechanics, Revised ed. (Kyoritsu, Tokyo, 1971); C. Kittel, Introduction to Solid State Physics, 7th ed. (John Wiley & Sons, New York, 1996).] (a) Use the Debye model to find the partition function Z, the Helmholtz free energy F, the entropy S, the internal energy E, and the heat capacity C_V at low temperatures. Hint: The lattice specific heat c_ph at low temperatures is proportional to T^3, where T is the temperature of the solid. (b) For metals the electronic specific heat c_e, which is proportional to T, dominates over the lattice specific heat at low temperatures. As the temperature is raised from T~0 K, the lattice specific heat increases more rapidly than the electronic specific heat. At what temperature does the lattice specific heat become equal to the electronic specific heat in magnitude? How is this temperature related to the Debye temperature and the Fermi temperature? (c) Explain the physical meaning of the Debye frequency ω_D, the Debye wave number k_D and the Debye temperature T_D. (d) Discuss the similarities and the differences between the Einstein model and the Debye model. Which model agrees well with experiment? 3. The photon gas may be treated in a manner quite similar to the phonon gas. There are only two differences. First, there are two polarization states for photons compared with three for phonons. Second, the dispersion relation (the relation between the energy and the momentum or between the frequency and the wave number) isω = ck, where c is the speed of light for photons whereas it is the speed of sound for phonons. Consider a photon gas in a box of volume V. The box is kept at temperature T. The chemical potential of the photon gas is zero. (a) Find the thermodynamic potential , Helmholtz free energy F, the internal energy E, the entropy S, the heat capacity C, and the number of photons N. (b) Find the equation of state in the form pV ~ N k_B T. (c) The universe started from a big bang about 10^10 years ago. Currently the universe is expanding and thus cooling adiabatically. In 1964, A. A. Penzias and R. Wilson first measured the temperature of the universe (for which they were awarded a Nobel prize in Physics in 1978). The most recent measurements by the COBE (Cosmic Background Explorer) satellite give T = 2.725 +/- 0.001 K. Find the number density of the photons. (What is the number of photons per cubic centimeter?) This is called the cosmic background radiation (CBR). 4. The 2001 Nobel Prize in Physics was awarded to three physicists “for the achievement of BoseEinstein condensation (BEC) in dilute gases of alkali atoms, and for early fundamental studies of the properties of the condensates.” Go to the “Educational features” and “Further Reading” in the website http://www.nobel.se and answer the following questions. (a) (b) (c) (d) (e) (f) Explain briefly what they did. What kind of atoms were used? What is the number density of the atoms when BEC was achieved? What kind of methods were used to cool the atoms? How does one measure the temperature of the atomic gas? What is the use of BEC?