ele12104-sup-0001-TableS1
advertisement
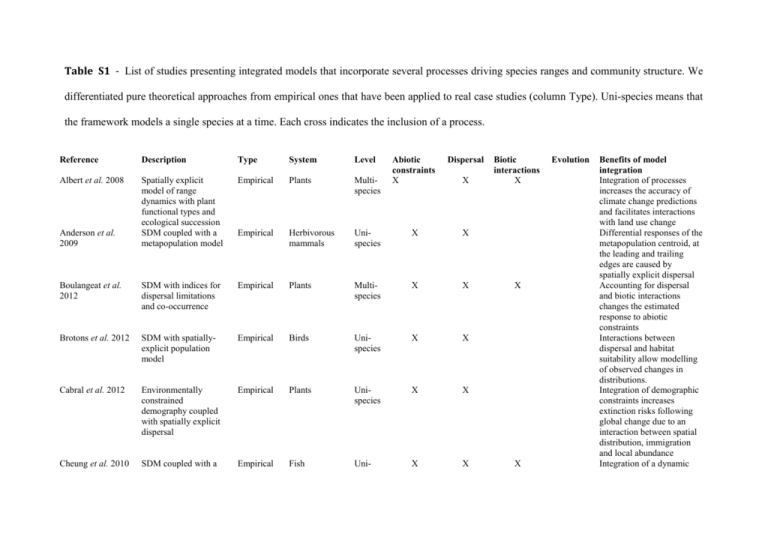
Table S1 - List of studies presenting integrated models that incorporate several processes driving species ranges and community structure. We differentiated pure theoretical approaches from empirical ones that have been applied to real case studies (column Type). Uni-species means that the framework models a single species at a time. Each cross indicates the inclusion of a process. Reference Description Type System Level Albert et al. 2008 Spatially explicit model of range dynamics with plant functional types and ecological succession SDM coupled with a metapopulation model Empirical Plants Multispecies Empirical Herbivorous mammals Boulangeat et al. 2012 SDM with indices for dispersal limitations and co-occurrence Empirical Brotons et al. 2012 SDM with spatiallyexplicit population model Cabral et al. 2012 Cheung et al. 2010 Anderson et al. 2009 Abiotic constraints X Dispersal Unispecies X X Plants Multispecies X X Empirical Birds Unispecies X X Environmentally constrained demography coupled with spatially explicit dispersal Empirical Plants Unispecies X X SDM coupled with a Empirical Fish Uni- X X X Biotic interactions X X X Evolution Benefits of model integration Integration of processes increases the accuracy of climate change predictions and facilitates interactions with land use change Differential responses of the metapopulation centroid, at the leading and trailing edges are caused by spatially explicit dispersal Accounting for dispersal and biotic interactions changes the estimated response to abiotic constraints Interactions between dispersal and habitat suitability allow modelling of observed changes in distributions. Integration of demographic constraints increases extinction risks following global change due to an interaction between spatial distribution, immigration and local abundance Integration of a dynamic spatially-explicit larval dispersal model species bioclimate envelope model that includes larval dispersal driven by ocean current through an advection–diffusion– reaction model Cheung et al. 2012 Ecophysiological model for individual fish growth coupled with a dynamic species distribution model Empirical Fish Unispecies X X Dullinger et al. 2012 Space and demography explicit model of range dynamics Empirical Plants Unispecies X X Engler & Guisan 2009 Species distribution model coupled with a spatially explicit dispersal model (cellular automaton) Empirical Plants Unispecies X X Kearney et al. 2009 Bio-energetic model of egg tolerance to desiccation with response to selection SDM coupled with a stochastic population model Empirical Mosquitoe larvae Unispecies X X Empirical Plants Unispecies X X Geneticecophysiological model for individual Empirical Trees Unispecies X Keith et al. 2008; Fordham et al. 2012 Kramer et al. 2008; Kramer et al. 2010 X X Maximum body weight of fish is expected to decrease due to the joint effect of a change in species distribution and in physiology, due to warmer and less oxygenated oceans The comparison to SDMs reveals that a majority of species will not be able to track suitable habitats in mountainous areas, creating an extinction debt The impact of realistic dispersal scenarios on range shifts depends on the landscape configuration (e.g. mountains or rivers) and the pace of climate change Identification of key traits responsible for evolutionary change under climate warming Extinction risk following global change depends on the dispersal mode, life history type, disturbances and distribution pattern Potential evolutionary response to climate change depends on generation time trees Meier et al. 2012 Combination of SDM with forest gap model Empirical Forests Multispecies X X X Mokany et al. 2012 Combining correlative models of alpha and beta diversities with a neutral metacommunity model. SDM coupled with a metapopulation model Empirical Plant Multispecies X X X Empirical Ants Unispecies X X Smolik et al. 2010 SDM coupled with an interacting particle system Empirical Plant Unispecies X X Spooner et al. 2011 SDM model coupled with host–affiliate species–discharge relationships Empirical Fish Multispecies X Thomas et al. 2012 Eco-evolutionary modelling framework called adaptive dynamics Empirical Phytoplankton Unispecies X Roura-Pascual et al. 2009 X X and the density of mother trees, and therefore interactively on land-use Interspecific competition slows the response to climate change, especially for late successional species One of the few approaches integrating metacommunity modelling, dispersal and abiotic constraints. Demonstrate the influence of historical and environmental factors in shaping the distribution of the Argentine ant (Linepithema humile) in Spain Integrated model better fits invasion data than isolated models and provide a less biased estimation of both dispersal and habitat suitability parameters Demonstrate the potential of combining environmental niche models to host–affiliate relationships in order to predict coextirpations from global change Using the species' thermal tolerance curve allows to calculate species growth rates under any given environmental temperature while accounting for trait change on ecological time Williams et al. 2008 Dispersal-constrained SDM Empirical Plants Unispecies X X X Wintle et al. 2005 Metapopulation model coupled with landscape-level forest dynamics model Empirical Birds Unispecies X X X Pagel & Schurr 2012 Environmentally constrained demography coupled with spatially explicit dispersal Simulation Populationlevel Unispecies X X Duputié et al. 2012 Multiple trait model and demographic model over a continuous space and diffusive dispersal Theoretical Populationlevel Unispecies X X Gravel et al. 2011 Colonizationextinction dynamics on islands constrained by prey availability Evolutionary model of predator-prey dynamics with explicit gene flow Theoretical Food webs Multispecies Theoretical Predator-prey Multispecies Moorcroft et al. 2006 Diffusion model for forest trees interacting with pathogens Theoretical Forestpathogen Multispecies Norberg et al. 2012 Metacommunity model Theoretical Competition Multi- Holt & Barfield 2009; Holt et al. 2011 X X X X X X X X X X X X X scales Facilitates early detection of new populations before high abundance threatens native biodiversity Land-use practices (forestry) and community dynamics (succession) at one level propagates at higher levels and influences persistence An integrated statistical methodology allows a nonbiased characterization of the fundamental niche and more accurate predictions of range shift Accurate understanding of the multivariate selective pressure is necessary to determine whether species track optimum conditions by dispersal or locally adapt Regional food web structure constrains distribution and the local food web structure A complex interplay between predator's generality, gene flow and landscape configuration can either limit or expand prey range Enemy-victim interactions can speed up migration rates by several orders through a Janzen-Connell effect Complex interactions with species sorting, local adaptation and dispersal Atkins & Travis 2010; Schiffers et al. 2013 Spatially explicit allelic simulation model with seed and pollen dispersal species Theoretical Individual Unispecies X X X among the four processes. High genetic variance and low dispersal minimize extinction risks under climate change Complex interactions between dispersal, habitat heterogeneity and local adaptation limit potential evolutionary rescue in a climate change context 1 Albert C.H., Thuiller W., Lavorel S., Davies I.D. & Garbolino E. (2008). Land-use change and subalpine tree dynamics: colonization of Larix decidua in French subalpine grasslands. Journal of Applied Ecology, 45, 659-669. 2 Anderson B.J., Akçakaya H.R., Araújo M.B., Fordham D.A., Martinez-Meyer E., Thuiller W. & Brook B.W. (2009). Dynamics of range margins for metapopulations under climate change. Proceedings of the Royal Society of London B, Biological Sciences, 276, 1415-1420 3 Atkins K.E. & Travis J.M.J. (2010). Local adaptation and the evolution of species' ranges under climate change. Journal of theoretical Biology, 266, 449-457. 4 Boulangeat I., Gravel D. & Thuiller W. (2012). Accounting for dispersal and biotic interactions to disentangle the drivers of species distributions and their abundances. Ecol. Lett., 15, 584-593. 5 Brotons L., De Caceres M., Fall A. & Fortin M.J. (2012). Modeling bird species distribution change in fire prone Mediterranean landscapes: incorporating species dispersal and landscape dynamics. Ecography, 35, 458-467. 6 Cabral J., Jeltsch F., Thuiller W., Higgins S., Midgley G.F., Rebelo A., Rouget M. & Schurr F. (2012). Impacts of past habitat loss and future climate change on the range dynamics of South African Proteaceae. Diversity and Distributions. 7 Cheung W.W.L., Lam V.W.Y., Sarmiento J.L., Kearney K., Watson R., Zeller D. & Pauly D. (2010). Large-scale redistribution of maximum fisheries catch potential in the global ocean under climate change. Glob. Change Biol., 16, 24-35. 8 Cheung W.W.L., Sarmiento J.L., Dunne J., Frolicher T.L., Lam V.W.Y., Deng Palomares M.L., Watson R. & Pauly D. (2012). Shrinking of fishes exacerbates impacts of global ocean changes on marine ecosystems. Nature Clim. Change, advance online publication. 9 Dullinger S., Gattringer A., Thuiller W., Moser D., Zimmermann N.E., Guisan A., Willner W., Plutzar C., Leitner M., Mang T., Caccianiga M., Dirnböck T., Ertl S., Fischer A., Lenoir J., Svenning J.-C., Psomas A., Schmatz D.R., Silc U., Vittoz P. & Hülber K. (2012). Extinction debt of high-mountain plants under twenty-first-century climate change. Nat. Clim. Change, 2, 619-622. 10 Duputié A., Massol F., Chuine I., Kirkpatrick M. & Ronce O. (2012). How do genetic correlations affect species range shifts in a changing environment? Ecol. Lett., 15, 251-259. 11 Engler R. & Guisan A. (2009). MIGCLIM: Predicting plant distribution and dispersal in a changing climate. Diversity and Distributions, 15, 590-601. 12 Fordham D.A., Akcakaya H.R., Araujo M.B., Elith J., Keith D.A., Pearson R., Auld T.D., Mellin C., Morgan J.W., Regan T.J., Tozer M., Watts M.J., White M., Wintle B.A., Yates C. & Brook B.W. (2012). Plant extinction risk under climate change: are forecast range shifts alone a good indicator of species vulnerability to global warming? Glob. Change Biol., 18, 1357-1371. 13 Gravel D., Massol F., Canard E., Mouillot D. & Mouquet N. (2011). Trophic theory of island biogeography. Ecol. Lett., 14, 1010-1016. 14 Holt R.D. & Barfield M. (2009). Trophic interactions and range limits: the diverse roles of predation. Proceedings of the Royal Society B-Biological Sciences, 276, 1435-1442. 15 Holt R.D., Barfield M., Filin I. & Forde S. (2011). Predation and the evolutionary dynamics of species ranges. The American Naturalist, 178, 488-500. 16 Kearney M., Porter W.P., Williams C., Ritchie S. & Hoffmann A.A. (2009). Integrating biophysical models and evolutionary theory to predict climatic impacts on species' ranges: the dengue mosquito Aedes aegypti in Australia. Functional Ecology, 23, 528-538. 17 Keith D.A., Akçakaya H.R., Thuiller W., Midgley G.F., Pearson R.G., Phillips S.J., Regan H.M., Araújo M.B. & Rebelo T.G. (2008). Predicting extinction risks under climate change: coupling stochastic population models with dynamic bioclimatic habitat models. Biol. Lett., 4, 560–563. 18 Kramer K., Buiteveld J., Forstreuter M., Geburek T., Leonardi S., Menozzi P., Povillon F., Schelhaas M., du Cros E.T., Vendramin G.G. & van der Werf D.C. (2008). Bridging the gap between ecophysiological and genetic knowledge to assess the adaptive potential of European beech. Ecological Modelling, 216, 333-353. 19 Kramer K., Degen B., Buschbom J., Hickler T., Thuiller W., Sykes M.T. & de Winter W. (2010). Modelling exploration of the future of European beech (Fagus sylvatica L.) under climate change - Range, abundance, genetic diversity and adaptive response. Forest Ecology and Management, 259, 2213–2222. 20 Meier E.S., Lischke H., Schmatz D.R. & Zimmermann N.E. (2012). Climate, competition and connectivity affect future migration and ranges of European trees. Global Ecology and Biogeography, 21, 164-178. 21 Mokany K., Harwood T.D., Williams K.J. & Ferrier S. (2012). Dynamic macroecology and the future for biodiversity. Glob. Change Biol., 18, 3149-3159. 22 Moorcroft P.R., Pacala S.W. & Lewis M.A. (2006). Potential role of natural enemies during tree range expansions following climate change. Journal of theoretical Biology, 241, 601-616. 23 Norberg J., Urban M.C., Vellend M., Klausmeier C.A. & Loeuille N. (2012). Ecoevolutionary responses of biodiversity to climate change. Nat. Clim. Change, doi:10.1038/nclimate1588. 24 Pagel J. & Schurr F.M. (2012). Forecasting species ranges by statistical estimation of ecological niches and spatial population dynamics. Global Ecology and Biogeography, 21, 293-304. 25 Roura-Pascual N., Bas J.P., Thuiller W., Hui C., Krug R.M. & Brotons L. (2009). From introduction to equilibrium: reconstructing the invasive pathways of the Argentine ant in a Mediterranean region. Glob. Change Biol., 15, 2101-2115. 26 Schiffers K., Bourne E.C., Lavergne S.b., Thuiller W. & Travis J.M.J. (2013). Limited evolutionary rescue of locally adapted populations facing climate change. Philos. T. R. Soc. Lon. B., 368. 27 Smolik M.G., Dullinger S., Essl F., Kleinbauer I., Leitner M., Peterseil J., Stadler L.M. & Vogl G. (2010). Integrating species distribution models and interacting particle systems to predict the spread of an invasive alien plant. Journal of Biogeography, 37, 411-422. 28 Spooner D.E., Xenopoulos M.A., Schneider C. & Woolnough D.A. (2011). Coextirpation of host-affiliate relationships in rivers: the role of climate change, water withdrawal, and host-specificity. Glob. Change Biol., 17, 1720-1732. 29 Thomas M.K., Kremer C.T., Klausmeier C.A. & Litchman E. (2012). A Global Pattern of Thermal Adaptation in Marine Phytoplankton. Science, 338, 1085-1088. 30 Williams N.S.G., Hahs A.K. & Morgan J.W. (2008). A dispersal-constrained habitat suitability model for predicting invasion of alpine vegetation. Ecol. Appl., 18, 347359. 31 Wintle B.A., Bekessy S.A., Venier L.A., Pearce J.L. & Chisholm R.A. (2005). Utility of dynamic-landscape metapopulation models for sustainable forest management. Conserv. Biol., 19, 1930-1943.