Solving Limiting Reactant (Reagent) Problems
advertisement
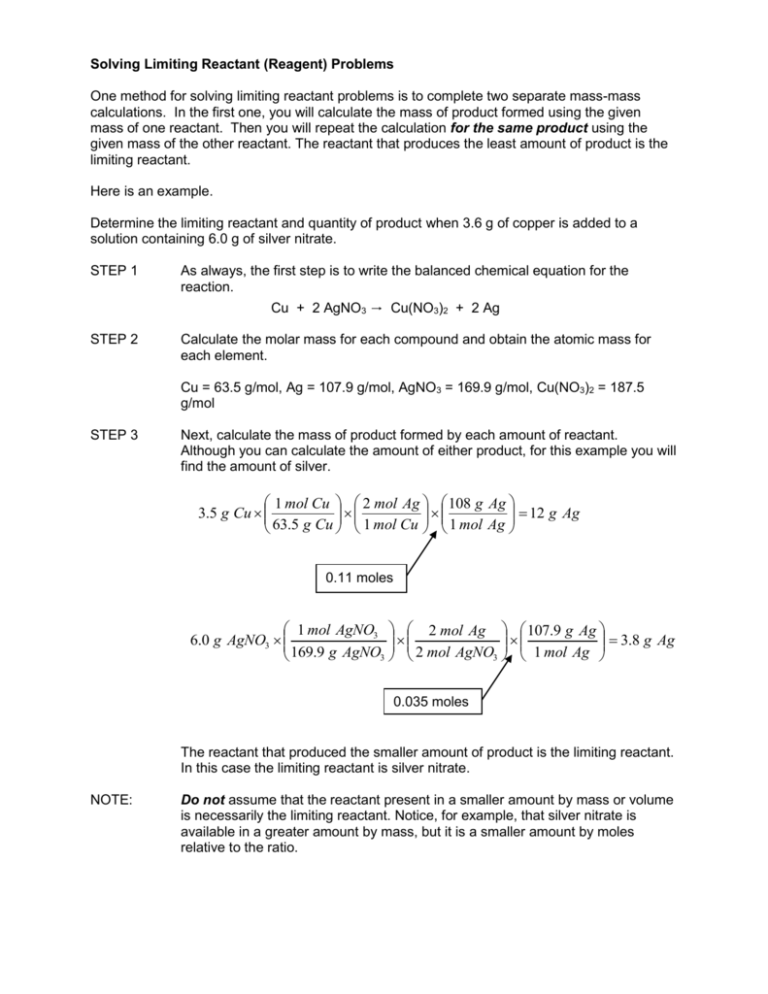
Solving Limiting Reactant (Reagent) Problems One method for solving limiting reactant problems is to complete two separate mass-mass calculations. In the first one, you will calculate the mass of product formed using the given mass of one reactant. Then you will repeat the calculation for the same product using the given mass of the other reactant. The reactant that produces the least amount of product is the limiting reactant. Here is an example. Determine the limiting reactant and quantity of product when 3.6 g of copper is added to a solution containing 6.0 g of silver nitrate. STEP 1 As always, the first step is to write the balanced chemical equation for the reaction. Cu + 2 AgNO3 → Cu(NO3)2 + 2 Ag STEP 2 Calculate the molar mass for each compound and obtain the atomic mass for each element. Cu = 63.5 g/mol, Ag = 107.9 g/mol, AgNO3 = 169.9 g/mol, Cu(NO3)2 = 187.5 g/mol STEP 3 Next, calculate the mass of product formed by each amount of reactant. Although you can calculate the amount of either product, for this example you will find the amount of silver. 1 mol Cu 2 mol Ag 108 g Ag 12 g Ag 3.5 g Cu 63.5 g Cu 1 mol Cu 1 mol Ag 0.11 moles 1 mol AgNO3 2 mol Ag 107.9 g Ag 3.8 g Ag 6.0 g AgNO3 169.9 g AgNO3 2 mol AgNO3 1 mol Ag 0.035 moles The reactant that produced the smaller amount of product is the limiting reactant. In this case the limiting reactant is silver nitrate. NOTE: Do not assume that the reactant present in a smaller amount by mass or volume is necessarily the limiting reactant. Notice, for example, that silver nitrate is available in a greater amount by mass, but it is a smaller amount by moles relative to the ratio.