Spectrochemical Methods Basics
advertisement
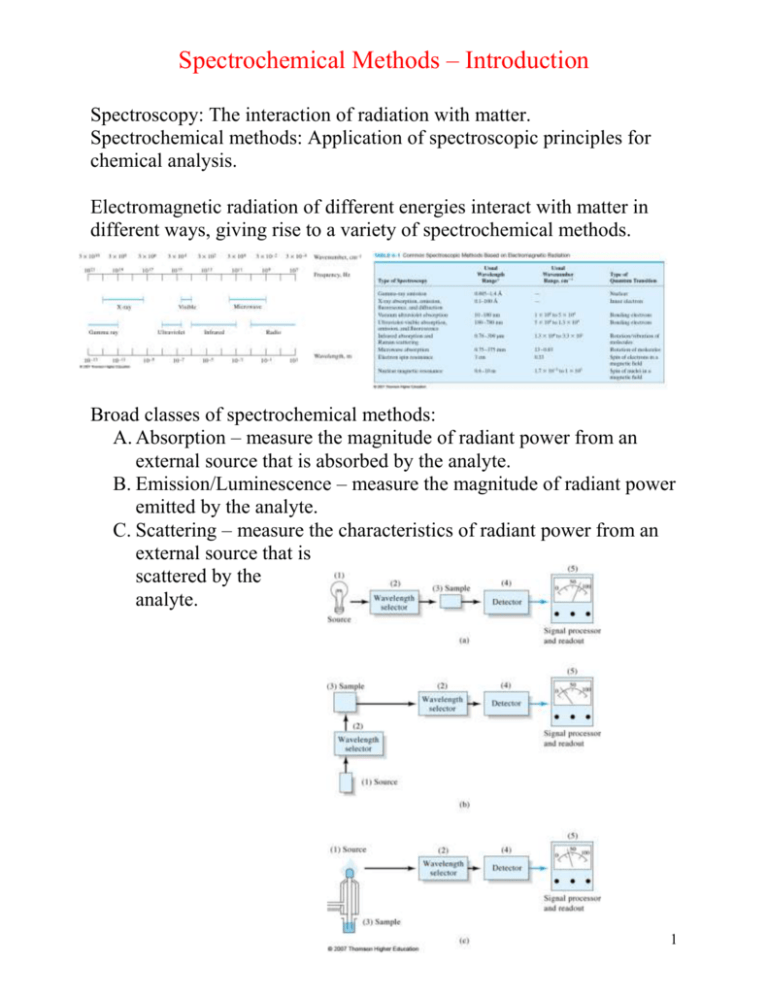
Spectrochemical Methods – Introduction Spectroscopy: The interaction of radiation with matter. Spectrochemical methods: Application of spectroscopic principles for chemical analysis. Electromagnetic radiation of different energies interact with matter in different ways, giving rise to a variety of spectrochemical methods. Broad classes of spectrochemical methods: A. Absorption – measure the magnitude of radiant power from an external source that is absorbed by the analyte. B. Emission/Luminescence – measure the magnitude of radiant power emitted by the analyte. C. Scattering – measure the characteristics of radiant power from an external source that is scattered by the analyte. 1 To understand optical spectrochemical methods, i.e. methods using the interaction of electromagnetic radiation with matter, a qualitative understanding of absorption and emission of electromagnetic radiation is required. The nature of electromagnetic radiation and its interactions with matter is discussed in Chapter 6. The wave model, which you should all be familiar with (Section 6B), explains phenomena such as: Wave superposition Diffraction 2 Also Refraction, Reflection, Transmission, Scattering all discussed in Section 6B. You are better off consulting your physics text for this information. The wave model fails to account for the absorption and emission of radiant energy; the basis for spectrochemical methods. Section 6C – quantum mechanical properties of radiation. EM radiation can also be viewed as a stream of particles called photons, the energy of which is proportional to frequency. Planck’s Law. 1. Atoms/Ions/Molecules can only exist in certain discrete states characterized by definite amounts of energy. When a species changes states, it absorbs or emits energy exactly equal to the energy difference between the states. 2. The frequency (ν) or wavelength (λ) of energy absorbed or emitted is related to the energy difference by Planck’s Law. 3 Although we will soon begin with a discussion of molecular (UV-Vis) absorption, look at the vapor phase emission spectrum of Na, along with its atomic energy level diagram. Higher energy orbital Lower energy orbital: Emission Lower energy orbital Higher energy orbital Absorption 4 The absorption spectra of molecules are very different from the absorption spectra of atoms. The energy of an atom Eatom = Eelectronic The energy of a molecule Emolecule = Eelectronic + Evibrational + Erotational Quantitative relationships of major classes of spectrochemical methods: Since we will start with two absorption spectrochemical methods (molecular and atomic UV-Visible absorption), a short discussion of the concentration relationship for this class of methods ensues. 5 Block diagram for UV-Vis molecular absorption instrument: Focusing in on the sample (cuvet) with pathlength b: Questions/Problems Chapter 6: 1f,g,l,m, 2-4, 7, 8, 14-17 6