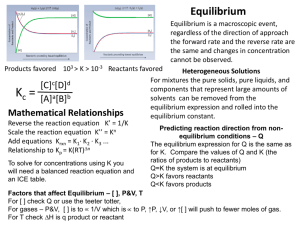
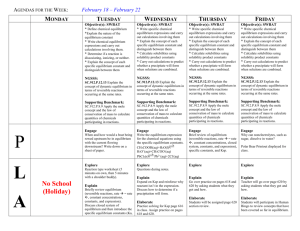
CHEMICAL EQUILIBRIUM REVIEW SHEET
advertisement

CHEMICAL EQUILIBRIUM REVIEW SHEET 1. A reaction is at equilibrium if the __________ of the forward reaction is __________ to the __________ of the reverse reaction. The _________________ of the reactants and products remain __________. 2. Describe how each of the following scenarios establishes equilibrium. a. ~20 mL of ethanol is placed into a 250 mL Florence flask and stoppered. b. A large pile of solid copper (II) chloride is placed into 50 mL of water. 3. Two stoppered flasks containing liquids are placed in front of you. When you open flask A, no odor is apparent upon wafting. When you open flask B, a familiar sweet odor is apparent upon wafting. What is the difference between the equilibrium vapor pressures of these two compounds? Which is considered volatile and nonvolatile? 4. Two liquids in separate beakers are placed in front of you. You measure the pH of both and find that one is near pH = 2 and one is near pH = 6. Since low pH means a higher [H+], which of the two acids is the stronger electrolyte? Which is the weaker electrolyte? How do you know? 5. For the equilibrium N2 (g) + 2 O2 (g) 2 NO2 (g), a. Write the equilibrium constant expression for the given equilibrium. b. Interpret the equilibrium arrow. c. Calculate and interpret K, given [N2] = 0.0040 M, [O2] = 0.0060 M, and [NO2] = 0.0150 M. 6. Using the equilibrium below and Le Chátelier’s Principle, predict and explain the resulting shift in equilibrium due to the “stresses” added. 2 B (s) + 3 H2 (g) 2 BH3 (g) Hf° = –2264.0 KJ a. The pressure of the container is increased. b. The reaction is cooled. c. The amount of hydrogen in the container was decreased. 7. Write a dissolution equation and a Ksp expression for a saturated solution of zinc bromate. 8. Calculate the Ksp of lithium hydrogen phosphite at 40°C if the solubility is 7.11 g in 100. g of water. 9. Calculate the mass of praseodymium hydroxide in 10.0 L of a saturated solution at 25°C if the Ksp = 3.39 x 10–24. 10. Does a precipitate form upon the mixing of 25.00 mL of 0.500 M yttrium chloride and 25.00 mL of 0.350 M sodium carbonate? The Ksp of yttrium carbonate is 1.03 x 10–31.