Ionic bonds gizmo
advertisement

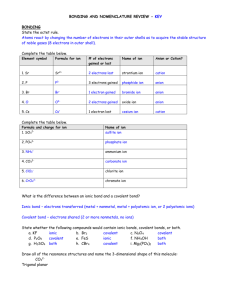
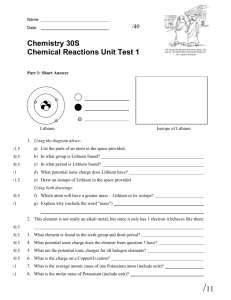
Name: IONIC BONDS GIZMO Although there are only approximately 90 naturally occurring elements on Earth, there are thousands of different substances that exist. The variety of different substances is a result of combining different elements, in different ratios, using different types of chemical bonds. In this activity, you will simulate ionic bonds between a variety of metal and nonmetal atoms. Go to www.explorelearning.com Login using the username: bannon and the password: science th Select the “8 Grade” tab Scroll down the page and find the Ionic Bonds gizmo and select “Launch Gizmo” Use the “Metals” drop down menu to select Sodium (Na) and the “Nonmetals” drop down menu to select Chlorine (Cl) Check the 3 boxes below these menus: Show Formula, Show Complete Compounds, and Show Charge Reduce the Speed bar (purple oval) to the lowest setting and press the Play button Drag and drop electrons from/to the atoms to create the correct ion for each atom If you believe that you have a group of ions that can bond to form a neutral compound, click the CHECK button If you need additional atoms (to form more ions) to create a neutral compound, you can ADD METALS or ADD NONMETALS Record the chemical formula for your neutral compound on the table below. Try the other metal/nonmetal combinations on the table. Be sure to record the chemical formula after you CHECK each combination. Metal - Amount Nonmetal – Amount Na O Mg Cl Al S Li O Be F Mg O Al N Mg P Chemical Formula Once you have completed all 7 metal/nonmetal combinations, call your teacher over to your computer to check. Your instructor will record your score here: _____ 1. Draw the ion that will be formed by the selenium atom shown below when it has a full set of valence electrons? 2. What is the best way to describe the transfer of electrons in an ionic bond? 3. A student is constructing an ionic bond between beryllium and chlorine and has reached the stage below (Beryllium just gave an electron to chlorine). What should the student do next and why? 4. Zinc phosphide, Zn3P2, is often used as a rat poison. Phosphorus has 5 valence electrons. How many valence electrons does each zinc atom lose? Explain how you were able to figure this out. 5. Lithium nitride has the chemical formula Li3N. Rubidium is in the same Group as lithium. What is the formula of rubidium nitride? Why is this? 6. Ionic bonds general create a neutral compound, such as Li3N or Lithium Nitride, how is this possible when both the Li ion and N Ion both would originally have a charge?