determination of the hydration number an ionic compound
advertisement

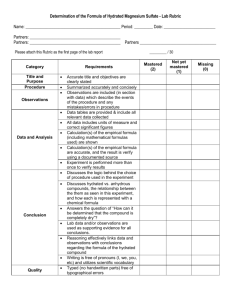
DETERMINATION OF THE HYDRATION NUMBER AN IONIC COMPOUND Procedure A crucible was cleaned and heated on a hotplate for 5 minutes and then allowed to cool. The mass of a clean, dry crucible was determined using a digital scale (±0.01g). Exactly 5.00 g of the hydrated copper (II) sulfate crystals was placed in the crucible. The hydrated crystals were observed and their appearance recorded. The crucible containing the hydrated copper (II) sulfate was heated on a hot plate to remove the water and form an anhydrous product. Its behaviour and appearance were observed and recorded. After about 10 minutes, the crucible and hydrate was removed from the hot plate, allowed to cool, and its mass determined This was repeated it irregular intervals until no significant change in mass was observed between successive weighings The appearance of the final product (anhydrous) was observed and recorded. The hydration number (lowest whole number ratio of moles of water to moles of anhydrous salt) was determined. Data Collection, Processing and Presentation Show ALL calculations needed to determine the hydration number of the hydrated copper (II) sulfate from your data. Conclusion and Evaluation State the formula of the hydrated copper (II) sulfate that you found from your data. Evaluate the accuracy and effectiveness of the experiment Suggest practical ways to imrpove the experiment other than performing the experiment yourself