Creating Retrovirus
advertisement
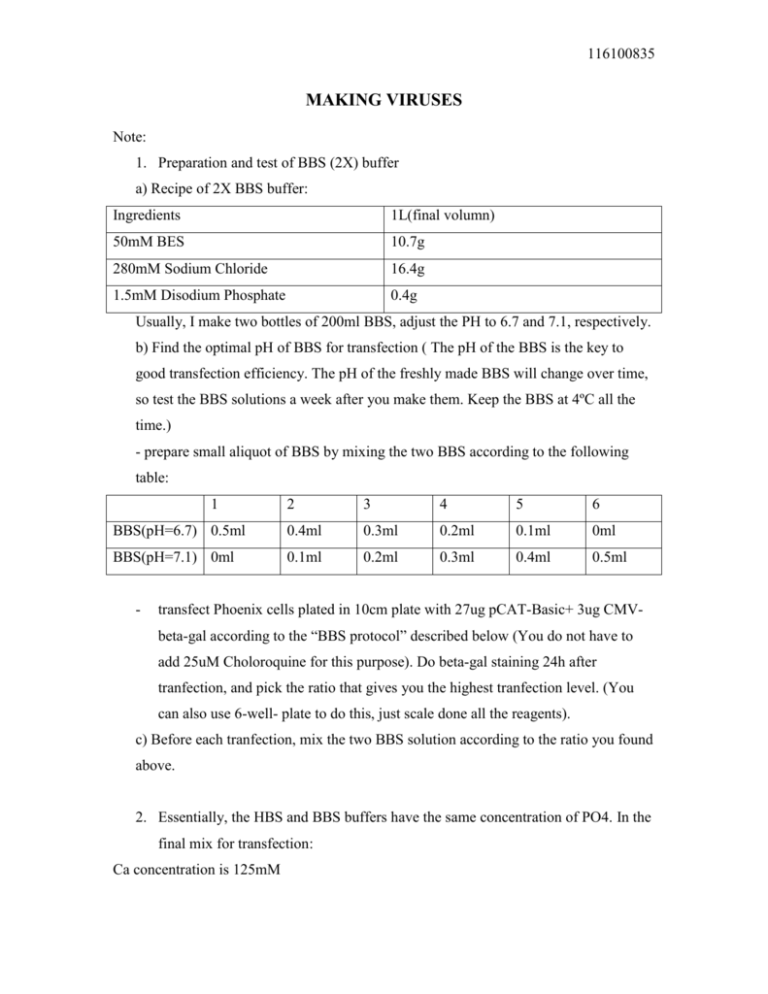
116100835 MAKING VIRUSES Note: 1. Preparation and test of BBS (2X) buffer a) Recipe of 2X BBS buffer: Ingredients 1L(final volumn) 50mM BES 10.7g 280mM Sodium Chloride 16.4g 1.5mM Disodium Phosphate 0.4g Usually, I make two bottles of 200ml BBS, adjust the PH to 6.7 and 7.1, respectively. b) Find the optimal pH of BBS for transfection ( The pH of the BBS is the key to good transfection efficiency. The pH of the freshly made BBS will change over time, so test the BBS solutions a week after you make them. Keep the BBS at 4ºC all the time.) - prepare small aliquot of BBS by mixing the two BBS according to the following table: 1 2 3 4 5 6 BBS(pH=6.7) 0.5ml 0.4ml 0.3ml 0.2ml 0.1ml 0ml BBS(pH=7.1) 0ml 0.1ml 0.2ml 0.3ml 0.4ml 0.5ml - transfect Phoenix cells plated in 10cm plate with 27ug pCAT-Basic+ 3ug CMVbeta-gal according to the “BBS protocol” described below (You do not have to add 25uM Choloroquine for this purpose). Do beta-gal staining 24h after tranfection, and pick the ratio that gives you the highest tranfection level. (You can also use 6-well- plate to do this, just scale done all the reagents). c) Before each tranfection, mix the two BBS solution according to the ratio you found above. 2. Essentially, the HBS and BBS buffers have the same concentration of PO4. In the final mix for transfection: Ca concentration is 125mM 116100835 PO4 concentration is 0.75mM Pka for CaHPO4 is 6.66 For VSVG virus: use 5ug VSVG + 20ug target retroviral vector. 1. BBS protocol (on 10cm plate) – The protocol I have been using -The day before transfection, plate 5-6×106 cells/10cm plate, so the cells will be 60-80% confluent on the day of transfection. (Note: Assume you start with a 90% confluent plate: split the cells 1:2 if you are plating the cells 16h before transfection, or split the cells 1:2.5 or 1:3 if you are plating the cells 24h before transfection. This will give you the right density for tranfection the next day.) -Prepare DNA solution Add 25ug retroviral DNA to 500ul 0.25M CaCl2, mix well (For VSVG virus, use 20ug of retroviral DNA and 5ug of VSVG vector, and use GP 293 cells). -Prepare 500ul of 2×BBS for each DNA solution -Add the 500ul DNA solution to the 500ul 2×BBS, mix immediately, and let stand for 10min at RT (do the mix one by one). -Remove media on the cells and add 9ml fresh media (10% FBS) + 25uM Choloroquine. -Add the above 1ml transfection mixture to the plate. -Incubate at 37ºC for 9-10 hours (incubation time is crucial), wash once with DPBS, and add fresh media. -Continue incubate at 37ºC for another 14 hours. -Remove the media, add fresh media. -Incubate for another 24h at 32ºC, collect the virus in a conical tube. Spin the virus at 3200RCF for 10 mins to remove cell debris, and freeze down the supernant at 80ºC. -Add fresh media, incubate for another 24h at 32ºC, and collect virus again after 24h. Spin the virus at 3200RCF for 10 mins to remove cell debris, and freeze down the supernant at -80ºC. 116100835 2. HBS protocol (on 10cm plate): This protocol is from Bennett. He uses half amount of the Ca comparing to the standard HBS protocol in the literature. -Dilute 20×CaCl2 (2.5M) 1:10. -Prepare DNA solution 250ul 2×CaCl2 (0.25M) 250ul DNA+H2O -Prepare 500ul of 2×HBS for each DNA solution -Add the DNA solution dropwise to 2×HBS, stand for 1min -Remove media and add the above1ml mixture to the cells -Incubate the plates at 37ºC for 15min. -Add back 9ml DMEM with 15%FBS, incubate at 37ºC for 16 hours -Remove the media and add fresh media, incubate for another 24h at 32ºC. -Collect virus. Add fresh media, and collect virus again after 24h.