Protocol
advertisement

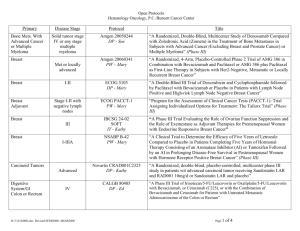
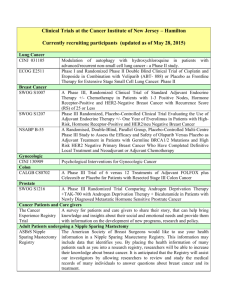
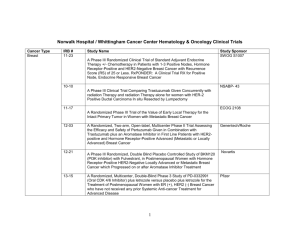
Open Protocols Hematology Oncology, P.C./Bennett Cancer Center Primary Disease Stage Protocol Locally Advanced or Metastatic ACORN AC01B07 PW - Mary Breast Breast Met or locally advanced Amgen 20060341 PW – Mary Title “A Double-Blind, Randomized Phase 2b Study of Sorafenib Compared to Placebo When Administered in Combination with Chemotherapy for Patients with Locally Advanced or Metastatic Breast Cancer that has Progressed During or After Bevacizumab Therapy” “A Randomized, 4-Arm, Placebo-Controlled Phase 2 Trial of AMG 386 in Combination with Bevacizumab and Paclitaxel or AMG 386 plus Paclitaxel as First-Line Therapy in Subjects with Her2-Negative, Metastatic or Locally Recurrent Breast Cancer” “An International, Randomized, Double-blind, Placebo-Controlled, Phase 2 Study of AMG 479 with Exemestance or Fulvestrant in Postmenopausal Women with Hormone Receptor Positive Locally Advanced or Metastatic Breast Cancer” Hormone Receptor Positive Locally Advanced or Met. Amgen 20070362 PW - Mary I-II ECOG 5103 DP - Mary “A Double-Blind III Trial of Doxorubicin and Cyclophosphamide followed by Paclitaxel with Bevacizumab or Placebo in Patients with Lymph Node Positive and High-risk Lymph Node Negative Breast Cancer” Adjuvant ECOG N063D DP – Mary (suspended as of 06/13/08) ALTTO: Adjuvant Lapatinib and/or Trastuzumab Treatment Optimisation Study: A Randomised, Multi-centre, Open-label, Phase III Study of Adjuvant Lapatinib, Trastuzumab, Their Sequence and Their Combination in Patients with HER2/ErbB2 Positive Primary Breast Cancer Breast Adjuvant Stage I-II with negative lymph nodes ECOG PACCT-1 PW - Mary “Program for the Assessment of Clinical Cancer Tests (PACCT-1): Trial Assigning Individualized Options for Treatment: The Tailorx Trial” (Phase III) Breast Observational Genentech AVF4349n Breast Breast Breast Breast D:\116097462.doc Revised 5JAN09 III IBCSG 24-02 SOFT IT - Kathy “An Observational Study of Treatment Patterns and Safety Outcomes for Metastatic or Locally Recurrent Breast Cancer (VIRGO)” “A Phase III Trial Evaluating the Role of Ovarian Function Suppression and the Role of Exemestane as Adjuvant Therapies for Premenopausal Women with Endocrine Responsive Breast Cancer” Page 1 of 4 Open Protocols Hematology Oncology, P.C./Bennett Cancer Center Breast Digestive System/GI Colon or Rectum Digestive Systems/GI Colon Digestive System/GI Colon Digestive System/GI Rectal Digestive System/GI Gastric or Esophagogastric Junction Genitourinary/GU Urothelial Tract or Bladder Genitourinary/GU Prostate D:\116097462.doc Revised 5JAN09 I-IIIA IV III II II or III Locally Advanced or Metastatic NSABP B-42 PW – Mary CALGB 80405 DP – Ed Accrual suspended 11/17/08 NCCTG N0147 DP – Ed “A Clinical Trial to Determine the Efficacy of Five Years of Letrozole Compared to Placebo in Patients Completing Five Years of Hormonal Therapy Consisting of an Aromatase Inhibitor (AI) or Tamoxifen Followed by an AI in Prolonging Disease-Free Survival in Postmenopausal Women with Hormone Receptor Positive Breast Cancer” (Phase III) “A Phase III Trial of Irinotecan/5-FU/Leucovorin or Oxaliplatin/5FU/Leucovorin with Bevacizumab, or Cetuximab (C225), or with the Combination of Bevacizumab and Cetuximab for Patients with Untreated Metastatic Adenocarcinoma of the Colon or Rectum” “A Randomized Phase III Trial of Oxaliplatin (OXAL) Plus 5-Fluorouracil (5-FU)/Leucovorin (CF) with or without Cetuximab (C225) after Curative Resection for Patients with Stage III Colon Cancer” E5202 DP – Ed “A Randomized Phase III Study Comparing 5-FU, Leucovorin and Oxaliplatin versus 5-FU, Leucovorin, Oxaliplatin and Bevacizumab in Patients with Stage II Colon Cancer at High Risk for Recurrence to Determine Prospectively the Prognostic Value of Molecular Markers” E5204 DP – Ed “Intergroup Randomized Phase III Study of Postoperative Oxaliplatin, 5Fluorouracil and Leucovorin vs Oxaliplatin, 5-Fluorouracil, Leucovorin and Bevacizumab for Patients with Stage II or III Rectal Cancer Receiving Preoperative Chemoradiation” Amgen 20060317 A Multicenter, Double-Blind, 3-Arm, Phase 1b/2 Study in Subjects with Unresectable Locally Advanced or Metastatic Gastric or Esophagogastric Junction Adenocarcinoma to Evaluate the Safety and Efficacy of First-line Treatment with Epirubicin, Cisplatin, and Capecitabine (ECX) plus AMG 102 Advanced or Metastatic Sanofi-Aventis EFC 6668 NC – Ed Randomized Study of Larotaxel + Cisplatin vs Gemzar + Cisplatin in 1st Line Treatment of Locally Advanced/Metastatic Urothelial Tract or Bladder Cancer” Metastatic COUGAR COU-AA-301 NC – Kathy A Phase 3, Randomized, Double-Blind, Placebo Controlled Study of Abiraterone Acetate (CB7630) Plus Prednisone in Patients with Metastatic Castration-Resistant Prostate Cancer Who Have Failed Docetaxel-Based Chemotherapy Page 2 of 4 Open Protocols Hematology Oncology, P.C./Bennett Cancer Center GYN Ovarian, Fallopian or Primary Peritoneal CA GYN Ovary, Peritoneal, Fallopian Tube GYN Ovarian, Primary Peritoneal Advanced Advanced Advanced Multiple Myeloma Multiple Myeloma D:\116097462.doc Revised 5JAN09 Genentech AVF4095g OCEANS DP – Sue “A Phase III, Multicenter, Randomized, Blinded, Placebo-controlled Trial of Carboplatin and Gemcitabine Plus Bevacizumab in Patients with Platinumsensitive Recurrent Ovary, Primary Peritoneal, or Fallopian Tube Carcinoma” GOG 0218 DP – Sue “A Phase III Trial of Carboplatin and Paclitaxel Plus Placebo versus Carboplatin and Paclitaxel Plus Concurrent Bevacizumab (NSC #704865, IND #7921) Followed by Placebo, versus Carboplatin and Paclitaxel Plus Concurrent and Extended Bevacizumab, in Women with Newly Diagnosed, Previously Untreated, Suboptimal Advanced Stage Epithelial Ovarian and Primary Peritoneal Cancer “ Genentech SHH4489g “A Phase II, Multicenter, Randomized, Double-Blind, Placebo-Controlled Trial Evaluating the Efficacy and Safety of GDC-0449 as Maintenance Therapy in Patients with Ovarian Cancer in a Second or Third Complete Remission” Genentech U4391g NC - Sue “A Phase III Multicenter, Open-Label Study of Rituximab Faster Infusion Time in Patients with Previously Untreated Diffuse Large B-Cell or Follicular Non-Hodgkin’s Lymphoma (RATE Trial) Advanced Millennium C05008 MB - Sue “A Phase I/II Study of VELCADE (Bortezomib), Dexamethasone, and Revlimid (Lenalidomide) (VDR) versus VELCADE, Dexamethasone, Cyclophosphamide, and Revlimid (VDR) in Subjects with Previously Untreated Multiple Myeloma” Advanced Novartis CZOL446EUS129 MB - Kathy GYN Ovarian Lymphoma Covance NV06-0039 DP – Sue Multi-Center, Randomized, Double-Blind, Phase III Efficacy Study Comparing Phenoxodiol (Oral Dosage Form) in Combination with Carboplatin versus Carboplatin with Placebo in Patients with PlatinumResistant or Platinum-Refractory Late-Stage Epithelial Ovarian, Fallopian or Primary Peritoneal Cancer Following at Least Second Line Platinum Therapy Met. or Locally Advanced “Bone Marker Directed Dosing of ZOMETA® (Zoledronic acid0 for the Prevention of Skeletal Complications in Patients with Advanced Multiple Myeloma” – (Z-Mark) Page 3 of 4 Open Protocols Hematology Oncology, P.C./Bennett Cancer Center IV Genentech AVF3991n ARIES DP - Ed IIIA/B Dartmouth Hitchcock D0410 DP - Ed “An Observational Study of Avastin (Bevacizumab) in Combination with Chemotherapy for Treatment of Metastatic or Locally Advanced and Unresectable Colorectal Cancer, Locally Advanced or Metastatic Non-Small Cell Lung (Excluding Predominant Squamous Cell Histology), or Locally Recurrent or Metastatic Breast Cancer” Note: Effective 10/20/08 only open for subjects with Stage IV NSCLC. “A National Web-Based Randomized Phase III Study of Erlotinib or Placebo Following concurrent Docetaxel, Carboplatin and Thoracic Radiotherapy in Patients with Inoperable Stage III Non-Small Cell Lung Cancer” E1505 DP - Ed “A Phase III Randomized Trial of Adjuvant Chemotherapy With or Without Bevacizumab for Patients with Completely Resected Stage IB (≥ 4cm) – IIIA Non-Small Cell Lung Cancer (NSCLC)” ImClone CP02-0452 PW - KD Randomized Phase III Study of Docetaxel or Pemetrexed with or without Cetuximab in Patients with Recurrent or Progressive Non-Small Cell Lung Cancer after Platinum-Based Therapy Observational Lilly H3E-US-B001 DP -Mary “Non-Small Cell Lung Cancer: The Impact of Ethnic Origin on Patients being Treated Second Line with Pemetrexed – an Observational Study” IB-IIIA OSI-774-302 RADIANT DP - Kathy Multiple Site NSCLC, Breast (The CRC portion closed to accrual 1/24/08) Pulmonary/Lung NSCLC Pulmonary/Lung NSCLC Adjuvant IB-IIIA Pulmonary/Lung NSCLC Pulmonary/Lung NSCLC IIIB, IV Pulmonary/Lung NSCLC Skin Cancer Melanoma T3 – T4 or N1 Melanoma D:\116097462.doc Revised 5JAN09 Stage IV Metastatic E1697 DP - Mary SYNTA 4783-08 SYMMETRY SD – Ed “A Multicenter, Randomized, Double-blind, Placebo-Controlled, Phase 3 Study of Single-agent Tarceva (erlotinib) Following Complete Tumor Resection with or without Adjuvant Chemotherapy in Patients with Stage IB-IIIA Non-Small Cell Lung Carcinoma who have EGF-positive Tumors” “A Phase III Randomized Study of Four Weeks High Dose IFN-a2b in Stage T2b N0, T3a-b, T4a-b N0, and T1-4, N1a, 2a, 3 (microscopic) Melanoma.” “A Randomized, Double-blind, Phase 3 Trial of STA-4783 in Combination with Paclitaxel versus Paclitaxel Alone for Treatment of Chemotherapy Naïve Subjects with Stage IV Metastatic Melanoma (SYMMETRY)” Page 4 of 4