Ferromagnetism of Nanoparticles and Secondary Crystal Structure
advertisement

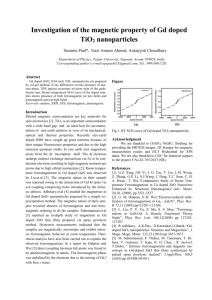
Ferromagnetism of Nanoparticles and Secondary Crystal Structure Yu.I. Vesnin yu_vesnin@ngs.ru It is known that magnetism is a universal property of substance [1]. In the work [2] it is shown that nanoparticles of inorganic substances of different classes (metals, oxides, various salts) reveal ferromagnetic properties. Suvbstances dia- and paramagnetic in massive state become ferromagnetic in superdispersed state (nanoparticles, size ~10-6 cm and less). Appearance of ferromagnetism of such particles is associated with various reasons (surface state of atoms, vacancies and other defects, magnetic anisotropy, Hund's rule etc.) [2]. In [3-5] the theory of secondary crystal structure (SCS) is developed as well as application examples of this theory in chemistry and physics of solids are given. According to the SCS theory, a crystal consists of elementary units of the size dm ~ 30 nm. This unit (the minimal crystal – a mic) is an analog of molecule – a giant crystalline molecule. A particle of smaller size (dsc 30 nm) is a subcrystal – an analog of molecule-radical, i.e. a free radical of crystal solid. It is known that magnetic susceptibility of radicals increases, and almost all known molecule-radicals are paramagnetic particles, i.e. they have magnetic moments [6]. Therefore, the crystal particles with sizes dsc < dm ~ 30 nm (subcrystals), like other radicals, should have magnetic moment as well. This moment takes place because of mass deficit (or quantity of atoms) relative to the mass of the elementary crystal unit (the main molecule). In this connection other properties of the crystal particle change as well [4]. It is known that magnetic structure of ferromagnetics consists of domains – magnetic ordered areas with macroscopic sizes. Each domain has a constant magnetic moment independent of an external field [1]. Without the external magnetic field these moments are mutually compensated, and magnetization is absent. In an ensemble of nanoparticles each particle can be a domain. Its magnetic moment is obtained owing to the transition to the state of the radical-molecule at the particle size dsc < 30 nm. Therefore, ferromagnetism can be expected for nanoparticles with the size dsc 30 nm. Magnetoactive form of substance appears here owing to the size effect [4]. In [2] it is shown that ferromagnetism of nanoparticles of various substances exists at temperatures 300 К and higher. It can be supposed that the ferromagnetic Curie point K will depend on of nanoparticle sizes with the maximum at a certain size. The ferromagnetism of nanoparticles has a principal meaning for the SCS theory. Earlier it was shown [4] that subcrystals (nanoparticles with sizes dsc < 30 nm) have the external force field with the extension in tens nm. The electron microscopic observations of crystal particles movement at annealing of discontinuous films evidence of the presence of these fields [7]. Ferromagnetism of nanoparticles allows supposing that observed force fields have magnetic nature. It can be checked in experiments on annealing of discontinuous films in a magnetic field (see Fig. 1). The movement character of particles will change depending on the field orientation. Determination of the magnetic nature of force fields of nanoparticles (subcrystals) can be of fundamental importance for chemical bond theory. Fig. 1 Movement of gold particles on a carbon substrate at t=264C under force magnetic fields of nanoparticles [7]. A similar experiment carried out in magnetic field will enable to determine the nature of nanoparticle force fields. References S.V. Vonsovsky. Magnetism. Nauka, Мoscow; 1971 (In Russian). A.Sundaresan, C.N.R. Rao. Nano Today, (2009), v. 4, p. 96. Yu.I. Vesnin. Jurn. Struct. Chem., (1995), v. 36, p. 724. Yu.I. Vesnin. Secondary structure and properties of crystals. SB RAS Edition Novosibirsk, 1997; www.nanometer.ru (In Russian) 5. Yu.I. Vesnin. Chemistry for sustainable development, (2000), v. 8, No. 1-2, p. 61. 6. V.N. Kondratyev. Free radicals as an active form of substance. Academy of Sciences UdSSR Edition, Мoscow; 1960 (In Russian). 7. W.B. Phillips, E.A. Desloge, J.G. Skofronik. J. Appl. Phys., (1968), v. 39, p. 3210. 1. 2. 3. 4.