RESEARCH INTERESTS
advertisement

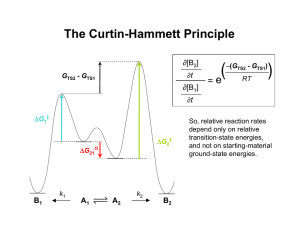
RESEARCH INTERESTS 1. Multicomponent reactions Multicomponent reactions are processes where three or more molecules react to form a single product. They are faster and cheaper than classical reactions, since they are done by just mixing compounds together in one vessel, without isolating any intermediate. The Yonemitsu condensation1 of indole, an aldehyde and Meldrum’s acid is an example of multicomponent reaction (Scheme 1). In its original version, only simple achiral aldehydes were used. R O O + H R N H O + O O MeCN, 25 °C O D,L-proline O N H R = Alkyl, Aryl O + H2O O Yield = 61 - 90 % Scheme 1 By working with the group of Pr. J. Sapi (University of Reims, France), we extended the original reaction to some chiral aldehydes such as Garner’s aldehyde (Scheme 2). 2 The condensation product can be selectively modified into some compounds with potential biological activities: pyrano- (1) and pyrrolidino-fused (2) tetrahydro--carbolines (helping memory effect), indolic analogue of azatoxin (3) (anticancer activity). 3 The stereogenic center of the aldehyde controls the configuration of all the stereocenters generated in the following steps. Therefore, the final products are obtained in high (> 90%) diastereomeric excess. N N H + N O boc O H O boc O O O MeCN, 25 °C O D,L-proline + O O O O N H several steps H N O O NH N H or NH N H CO2Me MeO2C OH O MeOOC or N N H O O MeO OMe OH (1) (2) (3) Scheme 2 1 Oikawa, Y.; Hirasawa, O.; Yonemitsu, O. Tetrahedron Lett. 1978, 19, 1759-1762. Dardennes, E.; Kovács-Kulyassa, Á.; Renzetti, A.; Sapi, J.; Laronze, J.-Y. Tetrahedron Lett. 2003, 44, 221-223. 3 (a) Sapi, J.; Laronze, J.-Y. ARKIVOC 2004, vii, 208-222; (b) Dardennes, E.; Kovács-Kulyassa, Á.; Boisbrun, M.; Petermann, C.; Laronze, J.-Y.; Sapi, J. Tetrahedron: Asymmetry 2005, 16, 1329-1339. 2 We further developed the trimolecular condensation by replacing Meldrum’s acid with other carbon acids and by varying the heterocycle (Scheme 3).4 In this case, the reaction requires the presence of TiCl4 and Et3N in stoichiometric amount. Respect to the original version, this titanium-promoted reaction has the advantage to allow the variation of all of the three reactants and a greater number of products can be obtained. If the carbon acid is asymmetric, the condensation product forms in high diastereomeric excess (>90 %). We are currently trying to make the reaction catalytic and enantioselective. Dr. A. Macchiarulo (University of Perugia, Italy) is also doing some docking studies in order to find the more likely biological properties of these compounds. O O Het + R1 H + R1 O TiCl4 (1 eq), Et3N (1 eq) OR2 OR2 Het CH2Cl2, 0°C-r.t. R3 R3 Yield = 41 - 90 % Het = Indoles, Pyrroles and Substituted Furans R1 = Alkyl, Aryl R2 = Me, Et, i-Pr R3 = COOR2, COMe, PO(OEt)2, NO2 Scheme 3 2. Study of reaction mechanisms We studied the mechanism of the titanium-promoted condensation in collaboration with Dr. A. Marrone (University of Chieti, Italy) by a combined theoretical and experimental investigation. Possible reaction pathways have been investigated by DFT calculations, whereas the reaction intermediates have been identified by NMR and UV-Vis spectrometry. The trimolecular reaction is probably a sequence of three simpler reactions (Scheme 4): a) Formation of the enolate ion; b) Knoevenagel condensation between the enolate ion and the aldehyde; c) Michael addition of the heterocycle to the Knoevenagel adduct. This study was crucial to understand the role of the metal and to tune the experimental procedure of the reaction. We are currently using this model to explain the observed diastereoselectivity. O H3CO 1 O OCH3 TiCl4, Et3N O H3CO IV Ti O 2 OCH3 O IV Ti O i-PrCHO H CO 3 Ti 3 OCH3 Indole O IV O H3CO OCH3 H N H Scheme 4 4 Renzetti, A.; Dardennes, E.; Fontana, A.; De Maria, P.; Sapi, J.; Gérard, S. J. Org. Chem. 2008, 73, 6824-6827.