AMIDE
advertisement

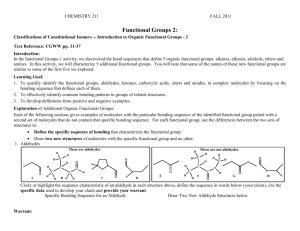
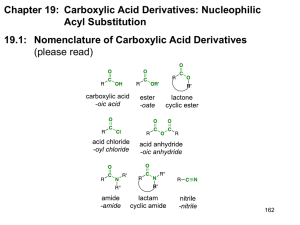
AMIDE While an ester can be thought of as a combination of a carboxylic acid and an alcohol, an amide is the combination of a carboxylic acid and an amine. Amides have the functional group: O || | -C-NAmides are classified as primary, secondary or tertiary depending upon how many carbons bond to the N. If there is one carbon, it is primary; if there are two , secondary and if there are three, tertiary. NAMING OF AMIDES 1) Locate the carbonyl group and name the parent carboxylic acid. 2) Replace the “oic acid” with the ending –amide. 3) A - Primary Amide – if there are two hydrogen atoms bonded to the nitrogen. This name needs no prefix. B – Secondary Amide – if there is one alkyl group attached to the nitrogen. Name and locate the alkyl group, use the letter N to indicate that it is bonded to the nitrogen atom. C – Tertiary Amide – if there are two alkyl groups attached to the nitrogen. Name and locate each alkyl group alphabetically, use the letter N to locate before each group. Eg. O || CH3-CH-C-NH2 | CH3 2-methylpropanamide O || CH3-CH2-CH2-C-NH-CH2-CH3 N-ethylbutanamide O || CH3-C-N-CH3 \ CH2-CH3 N-ethyl-N-methylethanamide EXERCISE 1. Name and classify the following amides. a) O b) O || || CH3-CH2-CH-CH2-C-NH2 CH3-CH-CH-C-NH-CH3 | | | CH2-CH3 CH3 CH3 c) O || CH3 –CH2-C-N-CH2-CH2-CH3 \ CH2-CH2-CH3 2. Give the condensed structural formulas for the following amides – classify each. a) N-methylpentanamide b) N,N-diethylbutanamide c) hexanamide PROPERTIES OF AMIDES Because the N atom is an electron withdrawing group, the C-N and H-N bonds are polar. As a result the physical properties of amides are similar to carboxylic acids. 1) Primary amides have two N-H bonds so they have even stronger hydrogen bonds than carboxylic acid. Secondary amides also have one N-H bond and experience hydrogen bonding. Tertiary amides do not experience hydrogen bonding. 2) Amides are water soluble. The longer the alkyl groups in the molecule, the lower the solubility in water. 3) Primary and secondary amides have higher melting points and boiling points than carboxylic acids. Many simple amides are solids at room temperature. REACTIONS Amides form from a reaction that is similar to an esterification. Instead of an alcohol an amine is used with a carboxylic acid and one molecule of water is created. The –OH group from the carboxylic acid and the -H from the –NH2 group will form a water molecule. O || CH3-CH2-C-OH + NH2-CH2-CH3 ---> propanoic acid aminoethane O || CH3-CH2-C-NH-CH2-CH3 + H2O N – ethylpropanamide Proteins are formed from amide bonds that form between amino acids. NH2-CH-COOH + NH2-CH-COOH + NH2-CH-COOH | | | CH3 CH2-OH CH2 –O NH2-CH-CO-NH-CH-CO-NH-CH-COOH | | | CH3 CH2-OH CH2 –O + 2 H2O - water is formed as the amide bond forms Questions 3. Give the name and the structure of the amide that forms between 3methylbutanoic acid and 2-aminopropane. 4. Show the amide formation between the amino acids glycine (2 amino ethanoic acid) and alanine (2 aminopropanoic acid).