of 4 - Clinical Departments
advertisement

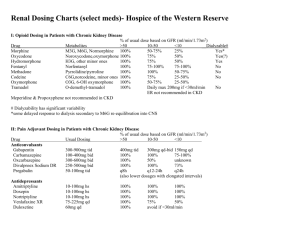
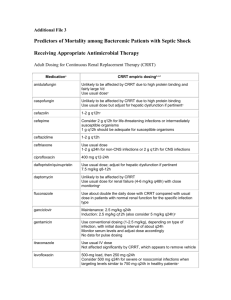
This form may be completed on line. Tab or move cursor to text field and type in text. *PHYSORDER* ADULT Diabetic Foot Infection Medication Orders For HIPAA Compliance reasons, this form IS NOT TO BE SAVED with patient information. Selecting the PRINT button will clear all information Page 1 of 4 Form Origination Date: 01/2013 Version: 1 from the note. Patient Name MRN PATIENT IDENTIFICATION LABEL ALLERGIES/DRUG SENSITIVITY: 1. 2. 3. 4. Weight: ________ kg Height: ________ cm Height: ________ in Ideal body weight (IBW): 50 kg + 2.3 kg for each in > 5 feet (male); 45.5 kg + 2.3 kg for each in > 5 feet (female) Adjusted body weight: IBW + 0.4 (actual weight - IBW) Diagnostic orders: see algorithm (link) Foot X-ray Culture if expressible exudate present (for moderate, severe or limb-threatening infections): Diabetic foot culture Document Pseudomonas risk (mark all that apply): Recent (within 90 days) ICU admission Immunosuppressive state / medications (see algorithm for examples) Document MRSA risk (mark all that apply): Resident of long-term care facility History of MRSA colonization ESRD / dialysis Hospitalized / institutionalized / Wound care in past 30 days Immunosuppressive state / medications IV drug abuse Home infusion therapy Recent antibiotics incarcerated 2 days out of past 90 days MILD (may treat as outpatient) - see algorithm (link): Choose from I or II I. No MRSA risk factors: Choose 1 of the following Cephalexin 500 mg PO Q ___ H Amoxicillin-clavulanate 875 mg PO Q ___ H II. MRSA risk factors: Choose 1 of the following A. No sulfa allergy: Sulfamethoxazole-trimethoprim DS ___ tab PO Q ___ H B. Sulfa allergy: Doxycycline 100 mg PO Q 12 H MODERATE - see algorithm (link): Diagnostic orders Consult General Surgery (if need for further debridement) Consult Wound Care Consult Infectious Diseases (if osteomyelitis present) Bone biopsy for culture CRP, low sensitivity ESR MRI of foot (consider if elevated CRP or ESR) MODERATE: NO Pseudomonal AND NO MRSA risk factors present: Choose 1 of the following A. Preferred regimen: Ampicillin-sulbactam ___ gram IV Q ___ H B. Penicillin allergy not anaphylaxis: Ertapenem ___ gram IV Q 24 H C. Penicillin allergy anaphylaxis: Vancomycin ___ mg (25 mg/kg) IV x 11 then ___ mg (15 mg/kg) IV Q __ H (consult pharmacy for additional dosing) AND Metronidazole 500 mg IV/PO Q 8 H (if gangrene present) Physician Signature Pager ID Date Time AM/PM This form may be completed on line. Tab or move cursor to text field and type in text. *PHYSORDER* ADULT Diabetic Foot Infection Medication Orders Page 2 of 4 Form Origination Date: 01/2013 Version: 1 For HIPAA Compliance reasons, this form IS NOT TO BE SAVED with patient information. Selecting the PRINT button will clear all information from the note. Patient Name MRN PATIENT IDENTIFICATION LABEL MODERATE: NO Pseudomonal AND NO MRSA risk factors present (CONT): Choose 1 of the following D. ESBL-producing organism isolated or history of: Meropenem ___ gram IV Q ___ H MODERATE: Pseudomonal risk factors but NO MRSA risk factors present: Choose 1 of the following A. Preferred regimen, including penicillin allergy not anaphylaxis: Cefepime ___ gram IV Q ___ H AND Metronidazole 500 mg IV/PO Q 8 H (if gangrene present) B. Penicillin allergy anaphylaxis: Aztreonam ___ gram IV Q ___ H AND Clindamycin 300 mg IV or PO Q 8 H C. ESBL-producing organism isolated or history of: Meropenem ___ gram IV Q ___ H MODERATE: NO Pseudomonal risk factors BUT MRSA risk factors present: Choose 1 of the following A. Preferred: Vancomycin ___ mg (25 mg/kg) IV x 11 then ___ mg (15 mg/kg) IV Q __ H (consult pharmacy for additional dosing) AND Ampicillin-sulbactam ___ gram IV Q ___ H B. Penicillin allergy not anaphylasis: Vancomycin ___ mg (25 mg/kg) IV x 11 then ___ mg (15 mg/kg) IV Q __ H (consult pharmacy for additional dosing) AND Ertapenem ___ gram IV Q 24 H C. Penicillin allergy anaphylaxis: Vancomycin ___ mg (25 mg/kg) IV x 11 then ___ mg (15 mg/kg) IV Q __ H (consult pharmacy for additional dosing) AND Aztreonam ___ gram IV Q ___ H AND Metronidazole 500 mg IV/PO Q 8 H (if gangrene present) D. ESBL-producing organism present or history of: Vancomycin ___ mg (25 mg/kg) IV x 11 then ___ mg (15 mg/kg) IV Q __ H (consult pharmacy for additional dosing) AND Meropenem ___ gram IV Q ___ H Physician Signature __________________________________________ Pager ID _________ Date ______ Time _____ AM/PM This form may be completed on line. Tab or move cursor to text field and type in text. *PHYSORDER* ADULT Diabetic Foot Infection Medication Orders Page 3 of 4 For HIPAA Compliance reasons, this form IS NOT TO BE SAVED with patient information. Selecting the PRINT button will clear all information Form Origination Date: 01/2013 Version: 1 from the note. Patient Name MRN PATIENT IDENTIFICATION LABEL MODERATE: BOTH Pseudomonal AND MRSA risk factors present: Choose 1 of the following A. Preferred regimen, including penicillin allergy not anaphylaxis: Vancomycin ___ mg (25 mg/kg) IV x 11 then ___ mg (15 mg/kg) IV Q __ H (consult pharmacy for additional dosing) AND Cefepime ___ gram IV Q ___ H AND Metronidazole 500 mg IV/PO Q 8 H (if gangrene present) B. Penicillin allergy anaphylaxis: Vancomycin ___ mg (25 mg/kg) IV x 11 then ___ mg (15 mg/kg) IV Q __ H (consult pharmacy for additional dosing) AND Aztreonam ___ gram IV Q ___ H AND Metronidazole 500 mg IV/PO Q 8 H (if gangrene present) C. ESBL-producing organism isolated or history of: Vancomycin ___ mg (25 mg/kg) IV x 11 then ___ mg (15 mg/kg) IV Q __ H (consult pharmacy for additional dosing) AND Meropenem ___ gram IV Q ___ H SEVERE OR LIMB-THREATENING: see algorithm (link) Diagnostic orders: Consult General Surgery (if need for further debridement) Consult Wound Care Consult Infectious Diseases Bone biopsy for culture CRP, low sensitivity ESR MRI of foot (consider if suspected soft tissue abscess or diagnosis of osteomyelitis uncertain) SEVERE OR LIMB-THREATENING ANTIBIOTIC ORDERS: Choose 1 of the following A. Preferred regimen, including penicillin allergy not anaphylaxis: Vancomycin ___ mg (25 mg/kg) IV x 11 then ___ mg (15 mg/kg) IV Q __ H (consult pharmacy for additional dosing) AND Cefepime ___ gram IV Q ___ H AND Metronidazole 500 mg IV/PO Q 8 H (if gangrene present) Physician Signature __________________________________________ Pager ID _________ Date ______ Time _____ AM/PM This form may be completed on line. Tab or move cursor to text field and type in text. *PHYSORDER* ADULT Diabetic Foot Infection Medication Orders For HIPAA Compliance reasons, this form IS NOT TO BE SAVED with patient information. Selecting the PRINT button will clear all information Page 4 of 4 Form Origination Date: 01/2013 Version: 1 from the note. Patient Name MRN PATIENT IDENTIFICATION LABEL SEVERE OR LIMB-THREATENING ANTIBIOTIC ORDERS (CONT) B. Penicillin allergy anaphylaxis: Vancomycin ___ mg (25 mg/kg) IV x 11 then ___ mg (15 mg/kg) IV Q __ H (consult pharmacy for additional dosing) AND Aztreonam ___ gram IV Q ___ H AND Tobramycin (once-daily dosing) 7 mg/kg ___ mg (round to nearest 10 mg; use adjusted body weight) IV over at least 30 minutes (larger doses may need to be infused over 1 hour) 2 x 1 (consult pharmacy for additional dosing) AND Metronidazole 500 mg IV/PO Q 8 H (if gangrene present) C. ESBL-producing organism isolated or history of: Vancomycin ___ mg (25 mg/kg) IV x 11 then ___ mg (15 mg/kg) IV Q __ H (consult pharmacy for additional dosing) AND Meropenem ___ gram IV Q ___ H For loading doses > 2 g, consult with pharmacy. If the patient has a history of vancomycin MIC 2 for S. aureus, consult Infectious Diseases or Antimicrobial Stewardship Team for alternatives. 2 Once-daily dosing should not be used in the setting of endocarditis, pregnancy, CrCl <50 ml/min, or in patients with significant burns. In these situations, traditional dosing should be utilized (consult pharmacy for dosing recommendations). 1 Antibiotic dosing based on renal function (see also http://kdpnet.louisville.edu/renalbook/adult/): > 50 ≥ 30 to ≤ 50 ≥ 10 to ≤ 30 ≤ 10 or HD CVVH Amoxicillinclavulanate 875 mg PO Q12H 875 mg PO Q12H 500 mg PO Q12H 500 mg PO Q24H No data Ampicillin-sulbactam 3 grams IV Q 6H 3 grams IV Q8H 3 grams IV Q12H 3 grams IV Q24H 3 grams IV Q8H Aztreonam 2 grams IV Q6H 2 grams IV Q8H 2 grams IV Q12H 1 gram IV Q24H (after HD) 2 grams IV Q12H CrCl (mL/min) Cefepime 2 grams IV Q8H 2 grams IV Q12H 2 grams IV Q24H 1 gram IV Q24H (after HD) 2 grams IV Q12H Cephalexin 500 mg PO Q6H 500 mg PO Q12H 500 mg PO Q12H 500 mg PO Q12H 500 mg PO Q12H Clindamycin 300 mg PO/IV Q8H 300 mg PO/IV Q8H 300 mg PO/IV Q8H 300 mg PO/IV Q8H 300 mg PO/IV Q8H Doxycycline 100 mg PO Q12H 100 mg PO Q12H 100 mg PO Q12H 100 mg PO Q12H 100 mg PO Q12H Ertapenem 1000 mg IV Q24H 1000 mg IV Q24H 500 mg IV Q24H 500 mg IV Q24H No data Meropenem 1000 mg IV Q8H 1000 mg IV Q12H 1000 mg IV Q12H 1000 mg IV Q24H 1000 mg IV Q12H 1 DS PO Q12-24H No data Sulfamethoxazoletrimethoprim Tetracycline 1 DS 1-2 TAB PO Q12H 1 DS 1-2 TAB PO Q12H 1 DS PO Q12-24H 1 DS PO Q24H if on hemodialysis Not recommended if not on dialysis 250 mg PO Q6H 250 mg PO Q12H 250 mg PO Q24H 250 mg PO Q24H 15 mg/kg IV ≤ 10: dose based on levels Vancomycin+ 15 mg/kg IV Q8-12H 15 mg/kg IV Q12-24H Q24-48H HD: 15 mg/kg after each HD + Adjust dosing based on trough levels (goal 10-15 mcg/mL for empiric use, 15-20 mcg/mL for confirmed MRSA) 15 mg/kg Q24H Physician Signature __________________________________________ Pager ID _________ Date ______ Time _____ AM/PM