Ch 13 Homework Key
advertisement
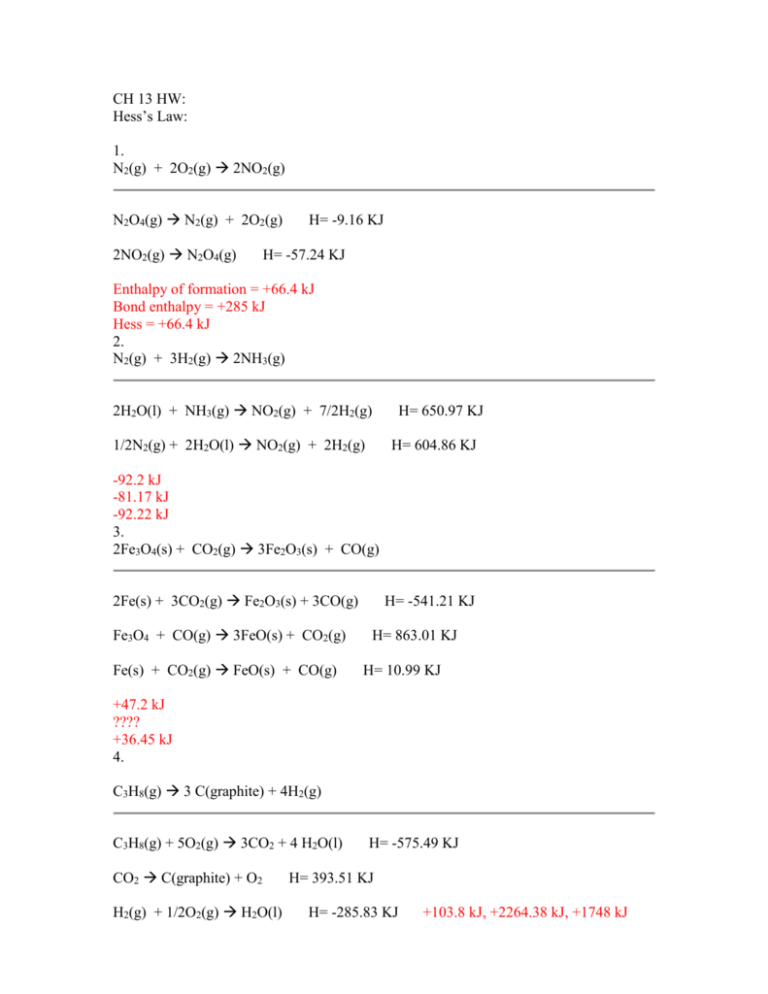
CH 13 HW: Hess’s Law: 1. N2(g) + 2O2(g) 2NO2(g) N2O4(g) N2(g) + 2O2(g) 2NO2(g) N2O4(g) H= -9.16 KJ H= -57.24 KJ Enthalpy of formation = +66.4 kJ Bond enthalpy = +285 kJ Hess = +66.4 kJ 2. N2(g) + 3H2(g) 2NH3(g) 2H2O(l) + NH3(g) NO2(g) + 7/2H2(g) 1/2N2(g) + 2H2O(l) NO2(g) + 2H2(g) H= 650.97 KJ H= 604.86 KJ -92.2 kJ -81.17 kJ -92.22 kJ 3. 2Fe3O4(s) + CO2(g) 3Fe2O3(s) + CO(g) 2Fe(s) + 3CO2(g) Fe2O3(s) + 3CO(g) Fe3O4 + CO(g) 3FeO(s) + CO2(g) Fe(s) + CO2(g) FeO(s) + CO(g) H= -541.21 KJ H= 863.01 KJ H= 10.99 KJ +47.2 kJ ???? +36.45 kJ 4. C3H8(g) 3 C(graphite) + 4H2(g) C3H8(g) + 5O2(g) 3CO2 + 4 H2O(l) CO2 C(graphite) + O2 H2(g) + 1/2O2(g) H2O(l) H= -575.49 KJ H= 393.51 KJ H= -285.83 KJ +103.8 kJ, +2264.38 kJ, +1748 kJ 5. C2H6(g) C2H2(g) + 2H2(g) C2H2(g) + 5/2O2(g) 2CO2(g) + H2O(g) H2O(g) H2(g) + 1/2O2(g) H= 241.82 KJ 2CO2(g) + 3H2O(g) C2H6(g) + 7/2O2(g) 311.4 kJ 297 kJ 310.74 kJ 6. P4(s) + 6Cl2(g) 4PCl3(g) P4(s) + 10Cl2(g) 4PCl5(g) PCl3(g) + Cl2(g) PCl5(g) -1148 kJ -1255.2 kJ -1148 kJ H= -1255.54 KJ H= 1427.8 KJ P4 = 6 single H= -1500 KJ white P H= -88 KJ 7. 2NO2(g) N2(g) + 2O2(g) N2(g) + 3H2(g) 2NH3(g) H= -92.22 KJ 2NO2(g) + 7H2(g) 2NH3(g) + 4H2O(l) H2O(l) H2(g) + 1/2O2(g) -66.4 kJ -284.5 kJ +66.4 kJ H= -1169.14 KJ H= 285.83 KJ 8. Which compound has the higher lattice energy: K2O or Na2O? KCl or SrCl2? Na2O smaller net radii; SrCl2 larger charges and more ions 9. Using the Kapustinskii equation, calculate the lattice energy for KCl and SrCl2 KCl = -672.38 kJ/mol, SrCl2 = -2134.62 kJ/mol Standard Bond Energies Single Bonds ΔH�* Single Bonds ΔH�* Multiple Bonds ΔH�* H–H 104.2 B–F 150 C=C 146 C–C 83 B–O 125 N=N 109 N–N 38.4 C–N 73 O=O 119 O–O 35 N–CO 86 C=N 147 F–F 36.6 C–O 85.5 C=O (CO2) 192 Si–Si 52 O–CO 110 C=O (aldehyde) 177 P–P 50 C–S 65 C=O (ketone) 178 S–S 54 C–F 116 C=O (ester) 179 Cl–Cl 58 C–Cl 81 C=O (amide) 179 Br–Br 46 C–Br 68 C=O (halide) 177 I–I 36. C–I 51 C=S (CS2) 138 H–C 99 C–B 90 N=O (HONO) 143 H–N 93 C–Si 76 P=O (POCl3) 110 H–O 111 C–P 70 P=S (PSCl3) 70 H–F 135 N–O 55 S=O (SO2) 128 H–Cl 103 S–O 87 S=O (DMSO) 93 H–Br 87.5 Si–F 135 P=P 84 H–I 71 Si–Cl 90 P≡P 117 H–B 90 Si–O 110 C≡O 258 H–S 81 P–Cl 79 C≡C 200 H–Si 75 P–Br 65 N≡N 226 H–P 77 P–O 90 C≡N 213 * Average Bond Dissociation Enthalpies in kcal mole-1 Lab: Hess’s Law Part 1: /30 1. Using a graduated cylinder, place 100.0 mL of water in a Styrofoam cup. 2. Obtain a cardboard lid containing 2 holes, a thermometer, and a stirring rod. 3. Record the initial temperature of the water. 4. Mass out 2.00g of sodium hydroxide and record. 5. Transfer the sodium hydroxide to the cup, place the cover on the top and stir. Record the maximum temperature reached (approximately 5 minutes). 6. Dispose of solution 1. Reaction: NaOH(s) Na+ + OHPart 2: 1. Make 100 mL of .5 M hydrochloric acid solution and add it to your Styrofoam cup. 2. Let this sit for 2-3 minutes and record the temperature. 3. Mass 2.00g of sodium hydroxide and record the mass. 4. Add the sodium hydroxide to the hydrochloric acid, cover, stir, and record the maximum temperature reached (approximately 5 minutes) 5. Dispose of solution 2. Reaction: NaOH(s) + H+ + Cl- H2O(l) + Na+ + ClPart 3: 1. Make 50 mL of 1M hydrochloric acid and add to the Styrofoam cup. 2. Make 50 mL of 1M sodium hydroxide. 3. Let both solutions sit for 2-3 minutes and then determine and record the temperature of both of the solutions. 4. Add the sodium hydroxide to the Styrofoam cup containing the hydrochloric acid. Stir this mixture and record the highest temperature reached (2-3 minutes). Reaction: Na+ + OH- + H+ + Cl- H2O(l) + Na+ + Cl- Calculations: Part 1: 1. Mass of 100 mL of water 2. Temperature change of the water 3. Heat produced (kJ) 4. Moles of NaOH 5. Heat produced/mol NaOH (kJ/mol) 6. ΔH1 = kJ/mol Part 2: 1. Mass 100 mL of hydrochloric acid. 2. Temperature change of solution. 3. Heat produced (kJ). 4. Moles NaOH 5. Heat produced/mol NaOH (kJ/mol) 6. ΔH2 = kJ/mol Part 3: 1. Mass of 100 mL of solution 2. Average initial temperature of solutions. 3. Temperature change of solution. 4. Heat produced (kJ). 5. Moles sodium hydroxide used. 6. Heat produced/mol NaOH (kJ/mol) 7. ΔH3 = kJ/mol Questions: 1. Write the net ionic equation for each of the 3 reactions on page 1 along with the appropriate enthalpy changes. NaOH(s) Na+ + OHNaOH(s) + H+ H2O(l) + Na+ OH- + H+ H2O(l) 2. Using Hess’s Law, compare the enthalpy change of reaction #2 to that of reactions #1 + #3. 3. Calculate the percent error using reaction #2 as the actual and the net of #1 and #3 as the experimental. 4. The dissolving of ammonium nitrate in water is endothermic. How could this be utilized for the treatment of athletic injuries? 5. If you repeated Part 1 of the lab but used twice as much sodium hydroxide, how would this affect the amount of heat produced? How would it affect the ΔH value? Would double assuming the solution is not saturated. Same because in KJ/mol 6. If double the amount of NaOH were used in Part 2, without changing any other concentrations or volumes, would this double the amount of heat produced in this reaction? Explain. No. HCl is limiting .1 L HCl x .5 mol HCl x 1 mol NaOH x 40 g NaOH = 2 g NaOH 1 L HCl 1 mol HCl 1 mol NaOH







