Clostridium difficile Toxin Gene Detection by DNA amplification
advertisement

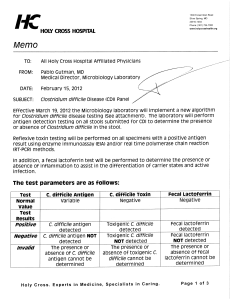
Laboratory Technical Bulletin Topic: Clostridium difficile Toxin Gene Detection by DNA amplification Test Name Test Code Method Changes Specimen Test Schedule C. difficile Toxin Gene CDIFF DNA Amplification This assay replaces the Clostridium difficile Toxin A+B enzyme immunoassay test. Formed stool specimens are not acceptable unless ileus is present. Specimens collected within 7 days of a previous specimen will be rejected. Raw stool or stool preserved in Cary-Blair-based transport medium. Transport refrigerated (2-8 °C). Monday through Sunday Introduction: Because of their low sensitivity, enzyme immunoassay (EIA) tests are no longer recommended for detection of C. difficile toxin in stool specimens.1 Recent reports indicate that molecular assays like the polymerase chain reaction (PCR) provide sensitivities and specificities comparable to toxigenic culture, the current "gold standard", and provide significantly shorter turnaround times.2 We compared the illumigene™ C. difficile (Meridian Bioscience Inc., Cincinnati, OH) loop-mediated isothermal amplification (LAMP) technology with our current EIA method (Premier™ Toxins A&B; Meridian Bioscience Inc., Cincinnati, OH) in order to evaluate the LAMP assay for possible use in our laboratory. The LAMP assay detects the pathogenicity locus (PaLoc) found in all toxigenic C. difficile strains. The EIA detects toxins A and B. We tested 95 non-formed raw stool specimens from hospitalized patients by the EIA and LAMP methods. Discrepant results were resolved by a PCR method that detects the tcdC regulatory gene. We obtained the following results compared to PCR: Test Sensitivity Specificity Current EIA test 64% 98% New LAMP test 100% 100% We concluded that the LAMP assay is superior to our current EIA method, provides similar TATs to the EIA, and increases sensitivity and specificity. Result Reporting: Results are reported as: Positive, Negative, or Inhibitory with recollection recommended. References: 1. Clinical Practice Guidelines for Clostridium difficile Infection in Adults: 2010 Update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA); Infection Control and Hospital Epidemiology May 2010, vol. 31, no. 5 2. Rapid and Sensitive Loop-Mediated Isothermal Amplification 1 (LAMP) Test for Clostridium difficile Diagnosis Challenges Cytotoxin B Cell Test and Culture as Gold Standard ; Tnorén, * I Alriksson, Josefin Andersson, Thomas Åkerlund, and Magnus Unemo . J. Clin. Microbiol. Vol. 49, No. 2 p. 710-711. Additional Information: Contact Donald Piper, Chief Medical Technologist, Microbiology, NorDx, at (207) 396-7816 or by email at piperd@mmc.org, or Monica Ianosi-Irimie, M.D., Ph.D., Laboratory Director, NorDx, at (207) 396-7809 or by email at ianosm@mmc.org