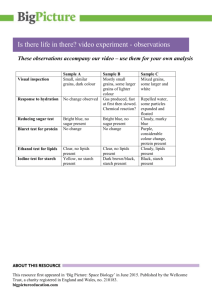
Željko Stojanović, Katarina Jeremić, Slobodan Jovanović Faculty of
advertisement
Željko Stojanović, Katarina Jeremić, Slobodan Jovanović
Faculty of Technology and Metallurgy. University of Belgrade, Belgrade, Yugoslavia
Synthesis of Carboxymethyl Starch
Carboxymethyl starch (CMS) was obtained under heterogeneous conditions as a product of the
reaction of starch and monochloroacetic acid (MCA) in the presence of sodium hydroxide. The
influence of the composition of the reaction mixture, the reaction time and temperature and the type
of starch being carboxymethylated on the degree of substitution, DS. and limiting viscosity number,
[η], was studied. The reaction temperature influenced the DS values, the highest values of the DS
being obtained when the carboxymethylation was performed at 58 ˚C. Increase of the molar ratio of
NaOH to an-hydroglycose units leads to an increase in DS, but only to certain extent. The values of
the DS and [η] of CMS increased almost linearly with increasing MCA amount in the reaction
mixture. The reaction mixture changes caused by adding water to ethanoi influenced the DS values
of the obtained CMS samples, the maximum DS value being obtained for CMS sample synthesized
in ethanol with 25% (v/v) water.
Keywords: Carboxymethylation; Heterogeneous conditions; Limiting viscosity number
1 Introduction
Awareness of the facts that existing quantities of fossil raw materials arc limited and that the
environment must be protected has significantly contributed to the reinterest in renewable raw
materials [1]. Consequently, there is an increasing number of papers dealing with the possibilities of
modifying biodegradable macromolecular substances based on renewable raw materials. The goal
of these modifications is to adjust the properties of macromolecular substances to different purposes
and to increase their consumption [2-5]. Starch has a special place among these polymers and,
among starch derivatives, car-boxymethyl starch (CMS) is of special interest.
Carboxymethyl starch is a water-soluble polysaccharide which is widely employed as an additive in
the paper industry [4], and as a thickener in the formulation of textile printing pastes [5]. The
application of CMS in enhanced oil recovery [5] is also growing. CMS is obtained as a product of
the reaction of starch, St-OH, and sodium monochloroacetate in the presence of sodium hydroxide.
This is a two-step reaction, the first step being the alka-lization of starch [6]:
In the second step etherification occurs:
Correspondence: Katarina Jeremic. c/o Faculty of Technology and Meallurgy, University of
Belgrade. Karnegijeva 4/IV. 11000 Belgrade, Yugoslavia. Phone: +381-11-3370-411, Fax: +38111-3370-387, e-mail: kjeremic@elab.tmf.bg.ac.yu
Additionally, the following side reaction is possible:
The carboxymethylation can be performed in water as a solvent [7-10] or in a water-miscible
organic solvent containing small amounts of water [11-13]. Carboxymethyl starches are reported to
have increased water solubility as the degree of substitution, DS, increases, so organic solvents are
to be used when a granular product is desirable.
The main reaction parameters which influence the carboxymethylation process are the solvent
system, solvent composition, concentration of NaOH and monochloroacetic acid (MCA),
temperature and duration of reaction [7-9,11,12]. In the present work, carboxymethylation was
performed in an ethanol/water mixture and the influence of the composition of the reaction mixture,
the reaction time and temperature and type of starch being carboxymethylated on the degree of
substitution and limiting viscosity number, [TJ], was studied.
2 Materials and Methods 2.1 Chemicals
The following types of starch were used for carboxymethylation: native starch, oxidized starch
(Starch Industry, Zrenjanin, Serbia, starch oxidized by hypochlorite solution, content of carboxyl
groups between 0.40-0.60 mass%) and starch enriched with amylose (BAGKF, Germany, 70%
amylose).
All the other chemicals used in this investigation (NaOH, CICH2COOH, CH3CH2OH, CuSO4,
FeCI3, EDTA, CaCI2,
NaCI, HCI. murexide) were produced either by Carlo Erba or Zorka (Šabac, Yugoslavia).
2.2 Synthesis of carboxymethyl starch
The synthesis of CMS was performed by a partially variant of the method for carboxymethylation
of cellulose/ starch mixtures developed by Samer and Bochov[14].
The synthesis of CMS was carried out in two steps. In the first step, alkalization was performed by
mixing starch, ethanol and aqueous 11.5M NaOH solution at room temperature. The reaction
mixture was stirred for 20 min, then MCA. dissolved in 11.5 M NaOH solution (the molar ratio of
MCA and NaOH was always equal to 6.83), was added and the reaction mixture heated up to the
desired temperature and stirred at that temperature for a definite time. The synthesized CMS
samples were purified by dissolving in water, neutralization of the solution with dilute NaOH or
HCI solution and precipitation with ethanol. The precipitated CMS was filtered and dried under
vacuum at 40 °C.
2.3 Determination of the degree of substitution
The DS was defined as the molar ratio of CH2COONa groups to anhydroglycose units (AGU) of the
starch molecule. The method developed by Kessel[15] was used for the DS determination. In this
method of analysis, the CH2COONa groups of CMS are determined by precipitating them as copper
salt and back titrating the excess copper in the filtrate with ethylenediaminetetraacetate (EDTA)
using murexide as the indicator.
2.4 Limiting viscosity number determination
An Ubbelohde dilution viscometer was used for determining the limiting viscosity number of starch
in water and CMS in 1.0% (w/w) NaCI at 25 ˚C. The values of [η] were determined graphically in
the usual way [16].
3 Results
Three types of starch were used for carboxymethylation. They were characterized by determining
the values of their [η] and the obtained results are presented in Tab. 1 together with the
abbreviations used for these starches.
Tab. 1. Types, abbreviations and limiting viscosity numbers, [η], of the starches used for
carboxymethylation.
The optimization of the process of carboxymethylation was performed by varying the process
parameters such as reaction medium, starch : liquor ratio, concentration of NaOH and MCA,
temperature and duration of reaction. Each parameter was varied keeping other parameters constant
as shown in different sets of experiments A-J in Tab. 2. The influence of these parameters on DS
and [η] was followed experimentally. The reaction efficiency (RE) was also calculated as follows:
3.1 Influence of carboxymethylation time
The duration of carboxymethylation was varied by performing the reaction for 50, 100. 150 and 200
min. The values of DS and [η] for the obtained products are given in Fig. 1. It can be seen that [η]
and DS increase with reaction time to achieve an almost constant value after 50 min. An acceptable
explanation for the data scattering at the longer reaction times could not be found. All the other
reactions of carboxymethylation were carried out for 50 or 100 min. The reaction efficiency (see
Tab. 2) changed in a similar way as the DS in this set of experiments.
3.2 Influence of the temperature of carboxymethylation
The dependence of DS and [η] values on the reaction temperature for the samples obtained by
carboxymethy-lating native starch (CMNS) and starch enriched with amylose (CMSA) are shown in
Fig. 2 and for samples obtained by carboxymethylating oxidized starch (CMOS) in Fig. 3.
Fig. 1. Dependence of [η] and DS on carboxymethylation time for CMNS samples at 58 °C. Other
reaction conditions as shown in Tab. 2.
Fig. 2. Dependence of [η] and DS on reaction temperature for CMNS and CMSA samples. Other
reaction conditions as shown in Tab. 2.
Fig. 3. Dependence of [η] and DS on reaction temperature for CMOS samples. Other reaction
conditions as shown in Tab. 2.
The DS of CMNS increases with reaction temperature, approaching an asymptotic value at 80 °C.
The values of the DS of CMSA samples are higher than those of CMNS and CMOS samples and
show a similar trend with reaction temperature as does the DS of CMNS. The lowest values of the
DS were obtained for CMOS and they increased with temperature in the whole investigated range
of reaction temperatures.
The values of [η] change differently with reaction temperature for the investigated samples. This
dependence is approximately the same for CMNS and CMSA, but it is quite different from the
corresponding dependence for CMOS. The values of [η] for the first two samples abruptly decrease
at a reaction temperature of about 58 °C. The values of [77] for CMOS increase with temperature.
Interestingly, the value of [η] for all three samples is the same at a reaction temperature of 68 °C.
An abrupt decrease in [η] might indicate some conformational change of the CMNS and CMSA
macromolecules or their degradation, but no evidence for this is yet available.
Further investigations of starch carboxymethylation were carried out at 58 °C since the highest DS
value was obtained at this temperature for all the investigated samples under the stated experimental
conditions.
3.3 Influence of NaOH
Native and oxidized starch were used to investigate the influence of the amount of NaOH on the
carboxymethylation reaction. The values of the DS and [η] of car-boxymethylated samples of native
starch are shown as a function of the molar ratio of NaOH to AGU of tho starch samples,
n(NaOH)/n(AGU), in Fig. 4 and of car-boxymethylated samples of oxidized starch in Fig. 5.
As shown in Fig. 4, during the carboxymethylation of native starch the dependence of the DS and
[77] on tho n(NaOH)/n(AGU) ratio in the reaction mixture is present-
Fig. 4. Dependence of [η] and DS on the n(NaOH)/ n(AGU) ratio for CMNS samples at 58 °C.
Other reaction conditions as shown in Tab. 2.
Fig. 5. Dependence of [η] and DS on the n(NaOH)/ n(AGU) ratio for CMOS samples at 58 °C.
Other reaction conditions as shown in Tab. 2.
Tab. 2. Reaction parameters used in the crboxymethylation of starch and the reaction efficiency
(RE).
ed by a curve which goes through a maximum. A similar change of the DS during the
carboxymethylation of native starch was obtained by Khalil et al. [8]. They explained the obtained
result by assuming that the side reaction between NaOH and sodium monochloracetate (Eq. 3) became more significant with increasing NaOH content in the reaction mixture, decreasing in that way
the concentration of sodium monochloracetate in the reaction mixture. Consequently, lower values
of the DS of the synthesized samples were obtained. It can also be seen from Fig. 4 that the highest value for the DS was
obtained for an n(NaOH)/n(AGU) ratio of 2, for the highest value of [η] this ration was 1.2. These
results indicate that during the carboxymethylation of native starch with increasing
n(NaOH)/n(AGU) ratio, a significant degradation of the amylose and amylopectin macromolecules
occurs, and even intramolecular crosslinking might take place causing a decrease of the [η] values.
The values of the DS for ox-
idized starch increase with increasing n(NaOH)/n(AGU) ratio, while the [rj] values decrease (Fig.
5). The DS values increase more slowly than for native starch (Fig. 4) and do not go through a
maximum. This might be caused by partial neutralization of the added NaOH by acidic groups in
the oxidized sample. The difference in the dependency of [η] on the n(NaOH)/n(AGU) ratio
obtained during the carboxymethylation of oxidized compared to native starch is most probably due
to the fact that the primary structure of oxidized starch has already been destroyed and that the
depoiymerization reaction occurs much more rapidly from the very beginning of carboxymethylation.
The reaction efficiency for the carboxymethylation of NS (see Tab. 2) also goes through a
maximum and the explanation for decreasing values at the higher n(NaOH)/ n(AGU) ratio is the
same as for DS behaviour.
3.4 Influence of MCA content
Native starch was used for the investigation of the influence of the MCA content in the reaction
mixture on the DS and [η] values of synthesized CMS samples. In these experiments the molar ratio
of MCA to AGU. n(MCA)/ n(AGU), was varied from 0.5 to 3.0. The obtained results are shown in
Fig. 6. The values of the DS and [r]] of CMNS increase almost linearly with the MCA content in
the reaction mixture. The results from Fig. 6 also show that the DS values of carboxymethylated
native starch increase more rapidly than the [η] values. By comparing the values of [η] shown in
Fig. 6 with the values shown in Fig. 2 it may be concluded that significant CMNS degradation
occurs with increasing MCA content, at a high concentration of NaOH in the reaction mixture. The
reaction efficiency for this set of experiments (see Tab. 2) is relatively low, which is probably also due to the high concentration of NaOH, favoring the side reaction
(Eq. 3).
3.5 Influence of reactant concentration and reaction mixture
The results obtained by investigating the influence of reactant concentration on the values of the DS
and [η] of the synthesized CMNS are shown in Fig. 7. The amount of reactant during these
experiments of the carboxymethylation of native starch was constant, but the concentration of the
reactants was decreased by increasing the amount of solvent - ethanol. As may be seen in Fig. 7, the
largest degree of substitution of native starch was obtained when 3.0 cm3 ethanol was used per 1.0 g
of starch. It was attempted to investigate even higher concentrations of starch, but the reaction
mixture became one sticky piece after some time and stirring became impossible. Interestingly, the
decrease of DS with the increasing amount of solvent is not linear, it becomes less steep at higher
dilution. This might indicate that the dilution effect is partly compensated for by a higher selectivity
with respect to the desired reaction.
Fig. 6. Dependence of [η] and DS on the n(MCA)/n(AGU) ratio for CMNS samples at 58 °C. Other
reaction conditions as shown in Tab. 2.
Fig. 7. Dependence of [η] and DS on the V(ethanol)/ m{starch) ratio for CMNS samples at 58 °C.
Other reaction conditions as shown in Tab. 2.
Ethanol was chosen as the reaction medium for the carboxymethylation of starch since under these
conditions the granular structure of starch is sustained and CMS is obtained as a fine powder. It was
considered worthwile to investigate the influence of water content on the DS since water aids in the
swelling of starch granules, thus facilitating entry of the etherifying agent into the starch granules.
Thus, four syntheses of CMS were performed keeping the concentration of all the reactants constant
and only changing the reaction medium. In one case the reaction medium was pure water, in the
second pure ethanol and in the third and fourth the water content in the reaction
Fig. 8. Dependence of [η] and DS on the reaction mixture (water/ethanol) ratio. Other reaction
conditions as shown in Tab. 2.
mixture was 25 and 50% (v/v), respectively. The values of the DS and [r;] were determined for the
obtained samples and are presented in Fig. 8. The results show that the DS and [η] values of the
CMNS samples go through a maximum when the water content is increased. The maximum value
of the DS is located at approximately 25% (v/v) of water. A similar result was obtained for CMS
synthesized in a mixture of water and other organic solvents [11]. Granular structure of CMS was
obtained in all cases except for pure water.
Besides native starch, oxidized starch (CMOS) and starch enriched with amylose (CMSA) were carboxymethylated in pure water and undiluted ethanol. The obtained samples were characterized and
the results are listed in Tab. 3. As can be seen the starch type and its previous history have
practically no influence on the DS values when carboxymethylation is carried out in water as the
reaction medium. This is most probably the consequence of the fact that all the used starch types
transform into the same gel state already during aikalization in water and the carboxymethylation
reaction slows down in
Tab. 3. Values of [η] and DS of different carboxymethyl-starches obtained in ethanol and in water
as reaction medium. Other reaction conditions as shown in Tab. 2.
the same way. When carboxymethylation is performed in ethanol, starch maintains its globular
structure during the whole reaction. The globular structure depends on the starch type and its
previous history thus influencing carboxymethylation. Consequently, different DS values are
obtained for different starch types, although the reaction conditions are the same.
4 Conclusion
Carboxymethyl starch was obtained under heterogeneous conditions as a product of the reaction of
starch and monochloroacetic acid, MCA. in the presence of sodium hydroxide. In the present work,
carboxymethylation was performed in an ethanol/water mixture and the influence of solvent
composition, concentration of NaOH and MCA, temperature and duration of reaction and the type
of starch being carboxymethylated on the degree of substitution, DS, and limiting viscosity number,
[η], was studied. Three types of starch were carboxymethylated: native starch, starch enriched with
amylose and oxidized starch. The increase of reaction temperature increased the DS values for the
samples obtained by carboxymethylation of all starch types, the values of [η] for samples obtained
by carboxymethyiation of native starch (CMNS) and starch enriched with amylose (CMSA)
decreased abruptly at a reaction temperature of 58 °C, while for the samples obtained by
carboxymethylation of oxidized starch (CMOS) values of [η] increased with the reaction
temperature. An abrupt decrease in [η] might indicate some conformation-al change of CMNS and
CMSA macromolecules or their degradation, but no evidence for this is still available. Increase of
the molar ratio of NaOH to anhydroglycose units leads to a DS increase, but only to a certain extent,
the values of the DS and [η] of CMS incrased almost linearly with increasing MCA amount in the
reaction mixture. The reaction mixture changes caused by adding water to ethanol influenced the
DS values of the obtained CMS samples, the maximum DS value being obtained for CMS sample
synthesized in ethanol with 25% (v/v) of water. The starch type and its previous history have
practically no influence on the DS values when carboxymethylation is carried out in water as the
reaction medium while the opposite is true for carboxymethylation in ethanol as the reaction
medium.
Bibliography
[1] Perspektiven nachwachsender Rohstoffe in der Chemie, Ed. H. Eierdanz, VCH, Weinheim
1996.
[2] D. Braun and K. H. Bahlig: Herstellung und Eigenschaften von Cellulosebenzoat. Angew.
Makromol. Chem. 220 (1994), 199-207.
[3] V. Bojanić, S. Jovanović, R. Tabaković and /. Tabaković: Synthesis and Electrochemistry of
Grafted Copolymers of
Cellulose with 4-Vinylpyridine, 1-Vinylimidazole, 1-Vinyl-2-pyrrolidinone. and 9-Vinyicarbazole.
J. Appl. Poiym. Sci. 60 (1996), 1719-1725.
[4] G. Reinisch. U. Radios and B. Roatsch: Rationelle Starke-acetat-Synthesen. Angew. Makromol.
Chem. 233 (1995). 113-120.
[5] M. Esan, T. Brummer and F. Meuser. Chemische und ver-fahrenstechnische Gesichtspunkte zur
Herstellung von kationischer Kartoffeistarke durch Kochextrusion. Starch/ Starke48(1996). 131136.
[6] K. A. Finch: Chemistry and Technology of Water-Soluble Polymers. Plenum Press. New York
1983. pp. 321-329.
[7] A. Hebeish and M. I. Khalil: Chemical factors affecting preparation of carboxymethyl starch.
Starch/Starke 40 (1988), 147-150.
[8] M. I. Khalil. A. Hashem and A. Hebeish: Carboxymethyia-tion of maize starch. Starch/Starke
42 (1990), 60-63.
[9] A. Hebeish. M. I. Khalil and A. Hashem: Carboxymethyla-tion of starch and oxidized starches.
Starch/Starke 42 (1990), 185-191.
[10] A. A. Ragheb. H. S. E/-Say/ad and A. Hebeish: Preparation and characterization of
carboxymethylstarch products and their utilization in textile printing. Starch/Starke 49 (1997). 238245.
[11] D. Bhattacharyya. R.S. Singhal and PR. Kulkarni: A comparative account of conditions for
synthesis of sodium carboxymethyl starch from corn and amaranth starch. Carbo-hydr. Polym. 27
(1995), 247-253.
[12] C. J. Tijsen. H.J. Scherpenkate, E.J. Stamhuis and A. A. C. M. Beenackers: Optimisation of
the process conditions for the modification of starch. Chem. Eng. Sci. 54 (1999). 2765-2772.
[13] K. Kwon. J.H. Auh. J. W. Kirn, K.H. Park. C.H. Park and C.J. Ko: Physicochemical
properties and functionality of highly carboxymethylated starch. Starch/Starke 49 (1997), 499-505.
[14] J. Samerand K. Bochov: Deutsche Demokratische Repub-likPat. 158403(1983).
[15] H. Kessel: Bestimmung der funktionellen Gruppe und des durchschnittlichen
Substitutionsgrades von Carboxymethyl-starke. Starch/Starke 37 (1985), 334-336.
[16] K. F. Arndt and G. Müller: Polymercharakterisierung, Hanser Verlag, München 1996, p. 142.
(Received: March 13, 2000)
(Revision received: September 12. 2000)