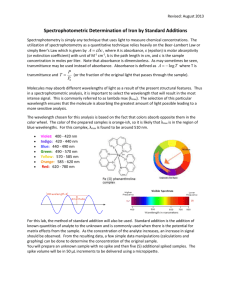
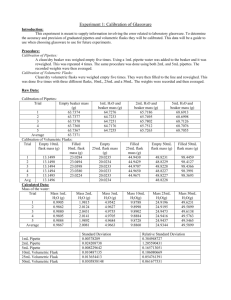
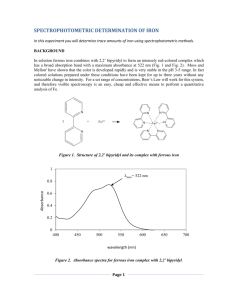
Vanadate-molybdate method
advertisement

Construction of phosphate Beer-Lambert Plot using standard phosphate solution KH2PO4 (50 µg P / 1mL) 3 point calibration - Label four 50mL volumetric flasks (blank, 2mL, 5mL, and 10mL of standard phosphate solution). - Place 10mL of Vanadate-molybdate reagent in each of the 50mL volumetric flasks. - Pipette 2mL (0.1mg P), 5mL (0.25mg P), and 10mL (0.5mg P) of the standard phosphate solution into the volumetric flasks, respectively. For blank, do not add any standard phosphate solution. - Add DI water into each flask (including blank) up to the 50mL mark. - Compute final concentrations for each flask (Eg. For 2mL of standard solution, 0.1mg P/0.05L = 2 mg P /L). - Wait for 10 minutes while reaction takes place. - Place each solution into a cuvette. - Blank as a blank - Measure absorbance for each cuvette (you can pick a wavelength of 400 nm for all measurements, since there will be no peak in the range between 400nm and 490nm). - Construct a Beer Lambert plot with the concentration on x-axis and absorbance on yaxis. - Save the Blank Solution for the sample determination. Determining phosphate concentration in samples - Place 25mL of a sample into 50mL volumetric flask. Add 10mL of Vanadate-Molybdate reagent and dilute to the mark with DI water (1 to 2 dilution of original sample). Wait for 10 minutes while reaction takes place. Place the sample solution into a cuvette. Blank Dark Measure absorbance for each cuvette (you have to pick the wavelength you used for Beer-Lambert plot). Find a concentration using BL plot and multiply it by 2 to get original concentration.