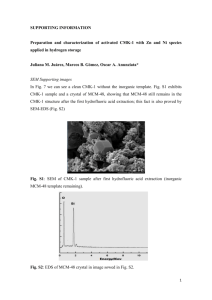
Supporting information The reaction between p

Supporting information
The reaction between p-NP and NaBH
4
without catalyst
Without catalyst, according to the same method, the time-dependent absorbance changes of the reaction between p-NP and NaBH
4
is measured. As shown in Fig.S1, it can be found that after adding to the NaBH
4
, the absorbance of solution increeased sharply. This phenomenon is common and can be ascribed to the reaction of p-NP and OH
-
, which is provided by the hydrolyzation of NaBH
4
. The experimental result indicates without catalyst, p-NP can not reduced to p-AP.
3.2
2.8
2.4
2.0
1.6
1.2
0 120
Time/s
240 360
Fig.S1. Time-dependent absorbance changes of the reaction between p-NP and
NaBH
4
without catalyst
The crystalline orientation of Au NPs
The crystalline orientation of Au NPs is investigated by TEM and XRD (Fig.S2 and S3). The nature of the diffraction spots (Inset of Fig.S2) clearly indicates that those NPs are [111] oriented single crystallinity. In the XRD pattern (Fig.S3), several sharp diffraction peaks are observed at 38.2°, 44.8°, 64.5° and 77.5°, which can be assigned to diffraction from the 111, 200, 220, and 311 planes of gold crystal, respectively. The intensity ratios of the 200 and 220 peaks to the 111 peak were much lower than the bulk value (0.34 and 0.24 versus 0.52 and 0.32), confirming that these
nanoparticles are dominated by [111] facets (Sun et al. 2005).
Fig.S2. TEM image of Au NPs. Inset is a convergent beam electron diffraction
(CBED) pattern.
Fig.S3. XRD pattern of Au NPs
Adsorption for p-NP on PEDOT powder
Before adsorption, PEDOT powder was prepared with HCl as doped acid and APS as oxidant via chemical polymerization. Then, 20 mg of PEDOT powder was added to
20 mL of p-NP aqueous solution (0.2 mMol/L) and stirred for 80 min. Samples were collected at the predetermined time intervals (0, 20, 40, 60 and 80 min, respectively), and the UV-Vis absorption spectra were measured. As shown in Fig. S4, it can be seen that the adsorption amount of p-NP on the PEDOT powder increases rapidly in the initial stage, then continues to increase slowly. The result indicates that PEDOT powder has good adsorption for p-NP, which may be ascribed to the secondly doped ability of PEDOT because p-NP is a weak acid. The adsorption of acid on PEDOT may be like that of other conducting polymer such as polyaniline (Mahanta et al.,
2009; Mahanta et al., 2009).
A
2.0
1.8
1.6
1.4
1.2
1.0
0.8
0.6
0.4
0.2
0.0
0min
20min
40min
60min
80min
300 400
Wavelength/nm
500
Fig.S4.
UV-Vis absorption spectra for p-NP on PEDOT powder at different period
References
Mahanta D, Madras G, Radhakrishnan S, Patill S (2009) Adsorption and desorption kinetics of anionic dyes on doped polyaniline. J Phys Chem B 113: 2293–2299.
Mahanta D, Madras G, Radhakrishnan S, Patill S (2008) Adsorption of sulfonated dyes by polyaniline emeraldine salt and its kinetics. J Phys Chem B 112:
10153–10157.
Sun XP, Dong SJ, Wang EK (2005) High-yield synthesis of large single-crystalline gold nanoplates through a polyamine process. Langmuir 21:4710–4712.