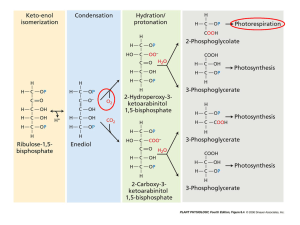
8 April
advertisement

Age of Rubisco 20 January TITLE PAGE SEPARATE The age of Rubisco Abstract Rubisco I (Form I of ribulose bisphosphate carboxylase/oxygenase) enables aerobic organisms to carry out the oxygenic extraction of carbon from air. The evolutionary history of oxygenesis is controversial. Evidence presented here suggests that Rubisco I organisms first appeared >2.83 Ga ago, and were abundant at 2.7-2.65 Ga ago. Carbon isotope evidence from ~2.7 Ga stromatolites in the Belingwe belt, Zimbabwe, from ~2.9 Ga stromatolites from Steep Rock, Ontario, Canada, and from ~2.9 Ga stromatolites from Mushandike, Zimbabwe implies that in all three localities the reef-building autotrophs did include organisms utilising Form I Rubisco (i.e aerobic oxygenic photosynthesisers). This evidence, though not conclusive, is consistent with other geochemical evidence for aerobic conditions in the presence of oxygen-rich waters where these stromatolites formed. Rubisco compensation constraints may explain the paradox that, despite the supposed evolution of oxygenesis as early as 2.9 Ga ago, the Late Archaean air was anoxic. Control of the atmospheric CO2:O2 ratio, and hence greenhouse warming, may have been linked to Rubisco I compensation limits and its specificity for CO2 over O2, in a bistable system that permits both anoxic and oxic stable states. Only when very high CO2 levels built up in the 2.3 Ga snowball may it have been possible for the atmosphere to move up the Rubisco I CO2 compensation line to an oxygen-rich system, limited by the O2 compensation barrier. Since then, Rubisco I compensation control may have helped sustain the atmospheric disproportion between O2 and CO2. 1 Age of Rubisco Introduction The air is a biological construction. Of the major gases, the dinitrogen burden is jointly managed by ammanox planctomycetes and by nitrifying and denitrifying bacteria, while dioxygen and CO2 are balanced by oxygenic photosynthesis. Only volcanic nitrogen (offset by lightning fixation) and the argon are abiotic. The history of the air is one of the Precambrian’s great puzzles (Macgregor, 1927). Did oxygenic photosynthesis begin late, around 2.3 Ga ago (Kopp et al., 2005)? Or was oxygen liberated by life much earlier, perhaps even as early as 3.8 Ga ago (Rosing and Frei, 2004)? Or is the answer to the puzzle somewhen between these extremes? There are four major forms (Ashida, 2005) of ribulose bisphosphate carboxylase/oxygenase, commonly termed rubisco. Form I, the focus of this study, is used by cyanobacteria, algae, plant chloroplasts, and many aerobic autotrophic bacteria. Form II is present in proteobacteria, mainly aerobic. Methanogenic archaea sequenced to date contain Form III Rubisco, and are anaerobes. Form IV Rubisco-like-proteins (RLP) are mainly found in anaerobes, such as Bacillus and green sulphur bacteria. RLP are not strictly Rubisco, as they are not proven to have carboxylase and oxygenase functions. Rubisco I is today the main interlocutor between carbon in the air and carbon in organic matter. Tcherkez et al, (2006) used transition state theory to show that in modern oxygenic photosynthetic organisms its specificity (preference for carbon dioxide over dioxygen) may be (in their words) "nearly perfectly optimised" to this task. The history of Rubisco I is presumably tightly linked to the evolution of oxygenic photosynthesis, which it supports. Here lies a puzzle. Although it is possible that local oxygen-rich conditions existed as early as >3.7 Ga ago (Rosing and Frei, 2004), there is strong evidence from the mass-independent fractionation (MIF) of sulphur isotopes that suggests the early Archaean atmosphere was anoxic. The MIF record suggests anoxia was maintained until about 3 Ga ago. Between 3 Ga and 2.6 Ga ago, the MIF record (Kasting and Ono, 2006) includes times when 33S was near 0‰, suggesting oxic conditions. Anoxic air returned until 2.45 Ga ago. Then a transition took place to oxygen-rich air by 2 Ga ago (Farquhar and Wing, 2003). However, the interpretation of the data is controversial, and there is evidence for MIF in modern volcanic deposits, despite the oxic air (Baroni et al., 2006; Savarino et al., 2003). Did oxygenesis only begin in the early Proterozoic as inferred by Kopp et al. (2005)? Or were there organisms producing oxygen in the Archaean, but without increasing the atmospheric oxygen burden beyond trace levels? These questions are more generally put as "how did the oxidation state of the atmosphere evolve?" To answer them, evidence is available in Archaean geology, mainly from the geochemistry of sediments (Canfield, 2005). Inferring the oxidation state of the air is welltrodden ground, but in most cases, interpretation of results remains controversial. As instrumentation improves, more results emerge. For example, the measurement of 33S, mentioned above, brought deep new insight into the history of atmospheric oxygen (Farquhar and Wing, 2003). Are there similar new tools to be found in the study of transition metal 2 Age of Rubisco isotopes? In addition to the geological evidence, molecular biochemistry is supporting sharp new attacks into the evolution of metabolism. These results need calibration against geological records, but may eventually allow contemporary Archaean ecologies to be mapped out. Archaean sedimentary environments are the best places to search for answers (Westall 2005). Much attention has been given to isotopic investigation of organo-sedimentary deposits in the Archaean. Among other lines of evidence, the MIF record of sulphides (Ono et al., 2003; Kasting and Ono, 2006) has already been mentioned. In the mid-Archaean, 3.2 Ga ago, the air had much more CO2 than today (Hessler et al., 2004). Possible direct evidence for oxygenic photosynthesis comes in the later Archaean (2.7 to 2.5 Ga), as biomolecular traces in bitumens from shales in the Pilbara, Western Australia have been interpreted as evidence for cyanobacteria (Brocks et al., 2003). Carbon isotopes in particular provide persistent signatures of life (Hayes, 2004, Schidlowski 1988). However, the interpretation of those signatures is complex and controversial. In many cases Archaean carbon isotope results have been taken to demonstrate that the material is the product of an oxygenic biosphere (e.g. Buick 1992; Schidlowski and Aharon, 1992; Schidlowski, 2002). Isotopic evidence has generally been interpreted as suggesting a stepwise history of metabolic evolution, with oxygenesis reshaping the global environment by the Late Archaean (des Marais et al., 1992; Kharecha et al., 2005). However, much of this work is based on the interpretation of coexisting organic carbon and carbonate. This makes assumptions about productivity: only in a highly productive biosphere would the cycling of organic carbon be able to control C-isotopes in carbonate. The isotopic discussion has mainly focussed on the link between oxygenesis and 13C in carbonates, rather than on an explicit search for isotopic signatures of autotrophy mediated by forms of Rubisco. In contrast to the views above, Kopp et al. (2005) sharply challenged the consensus that oxygenic photosynthesis evolved in the Archaean, proposing instead the hypothesis that it began in the Palaeoproterozoic, triggering the glaciation then. To summarise their views briefly, they reject all identification of oxygenic cyanobacteria prior to 2.3 Ga, broadly dismissing the biomarker evidence on varied grounds (e.g. as contaminants). They argue the C isotope evidence represents products of metabolic pathways other than oxygenic photosynthesis. They prefer to date the first oxygenic photosynthesis at 2.3 Ga, associated with massive Mn deposits in South Africa and global snowball glaciation. After 2 Ga ago there is agreement the atmosphere contained O2. There is therefore general consensus that oxygenic photosynthesis occurred after 2.3 Ga, but the date when the first oxygenesis began remains hotly contested. To address the dispute about the date of the first oxygenic photosynthesis (i.e. mediated by Rubisco I), we here return to detailed C isotopic studies in Archaean organosedimentary rocks. This work addresses these problems by searching for evidence of Rubisco I in unusually well-preserved late Archean reefs. Both organic carbon and carbonate are used to seek out this evidence. The most likely (though not unique) interpretation of our C isotopic results is that they are evidence for Rubisco 1 at ~2.9 Ga and ~2.7 Ga ago. If this is accepted, 3 Age of Rubisco our results support the view that oxygenic photosynthesis operated in the Late Archaean. If in turn this conclusion is accepted, then the next puzzle is to explain why the air remained anoxic until the Proterozoic. The second part of this study addresses this question. In general, the problem of understanding the history of atmospheric CO2 and O2 mixing ratios has mainly been seen in terms of inorganic chemistry. We put forward the hypothesis that the biochemistry of Rubisco I, including its compensation controls, may also have played an important role in atmospheric evolution. Isotopic signatures of Rubisco forms in organic matter in aerobic and anaerobic settings Carbon from the mantle enters the surface carbon cycle by degassing from volcanoes. Here it enters the atmosphere and thence the ocean by dissolution into seawater. Atmospheric CO2 equilibrates with the ocean surface, and enters the water as bicarbonate and carbonate ions, dissolved CO2, and carbonic acid. Some carbon is recaptured by alteration of lavas in the oceanic crust (Sleep and Zahnle, 2001) and thence subduction to the mantle. In the Archaean this process may have been a significant part of the global carbon budget. In carbonatised lenses in 3.5 Ga Pilbara basalts, hydrothermal carbonate occurs with 13C0‰ (Nakamura and Kato, 2004) suggesting that between mid-Archaean surface water and deep ocean the carbon isotope compositional gradient was small (Kharecha et al., 2005). Other carbon in the ocean enters organic matter. In the modern ocean, most carbon capture into organisms is carried out with the involvement of one of the forms of Rubisco, mainly Rubisco I in oxygenic aerobes. Life preferentially captures 12C-rich carbon: thus deposited organic carbon has markedly negative 13Creduced (Schidlowski, 1988). Modern organisms that fix carbon by Rubisco I (all of which are aerobes) typically have an isotope effect Product = reactant - of about 20-30‰; those that use Rubisco II have about 22‰, for those using the Acetyl-CoA pathway 15-36 ‰, and for those using the reductive or reverse tricarboxylic acid (TCA) path is 413‰, and for the 3-Hydroxypropionate cycle 0‰ (Hayes 2001). These are broad generalisations that depend on ambient conditions. Modern oxygenic phytoplankton that use Rubisco I (cyanobacteria and eukaryotic algae) do so in aerobic conditions with abundant ambient CO2, with 13CCO2 around -8‰ in air, or -7‰ in water. These modern phytoplankton typically show a 13Creduced range of –34‰ to –17‰ (Erez et al., 1998; Goericke et al., 1994; Guy et al., 1993). This implies in the range 28 to 10 ‰. In conditions of abundant CO2, carbon isotope fractionation by Rubisco I appears to be linearly correlated with CO2/O2 specificity (Tcherkez et al., 2006). Organisms with highly CO2 specific Rubiscos show larger fractionation. If the specificity (i.e. ratio in which CO2 is selected over O2 30‰. However, if specificity is below 10, then the effect drops towards 20‰. This is at 25oC. Specificity itself has an Arrhenius-type temperature dependency, with high CO2 specificity at low temperatures (say 5oC) and low specificity at, say, 60oC (Tcherkez et al., 2006). 4 Age of Rubisco Anoxygenic Rubisco II organisms (also aerobes) such as Rhodospirillum rubrum have a smaller carbon isotope effect. They may have evolved prior to oxygenic Rubisco I cells (Nisbet et al., 1995). Typically, 13Creduced is around –23 to –20‰ (Guy et al., 1993). In microbial mats, this smaller carbon isotope effect may in part be because of prior competition by Rubisco I cells. Within microbial mats, overlying cyanobacteria have first access to CO2. Deeper in the mat, limitation of CO2 supply occurs. In conditions of low CO2-availability or shortage, the fractionation is less. Shortage may force Rubisco II bacteria to be less selective against the thermodynamically unfavoured 13C, which will constrain 13Creduced. Among anaerobes that use Rubisco III or Rubisco IV (note that in these the function of the rubisco is poorly understood), the isotopic effect of carbon capture may vary widely. Methanogenic archaea (acetyl Co-A path) sharply fractionate carbon (Hayes 2004) especially if there is repeated carbon cycling in the biological community. Archaean and Proterozoic organic carbon with C isotopes lighter (more negative) than 13Creduced –35 ‰ is thus most probably interpreted as organic debris from methane cycling (e.g. Hayes, 1994). However, it should be remembered that carbon in anammox planctomycetes can have 13Creduced –47‰ (Schouten et al., 2004). In contrast, tricarboxylic acid cycle (TCA) bacteria (e.g. aquifecales and desulphobacters) can have low selectivity for carbon isotopes (Londry and Des Marais, 2003, Londry et al., 2004). The Rubisco I signature in carbonate minerals On the modern earth, the association of carbonate (especially calcite) with 13Ccarb around 0‰ and organic matter with 13Creduced in the range –25 to –30‰ is interpreted as the record of an atmosphere/ocean system in which biological productivity is dominated by oxygenic photosynthesis (Rubisco I). The air has 13C around –8‰. From ambient CO2 in the air, and from CO2 dissolved in seawater (which is slightly enriched in 13C compared to air), Rubisco I's carbon isotope effect then selectively extracts 12C into organic matter (13Creduced around –25 to –30‰). Carbonate precipitated in the same system is thus correspondingly enriched, more positive (13Ccarb around 0‰). Many Phanerozoic strata, in broadly oxic (or at least non-reducing) facies, contain the association of C-isotopically heavy stromatolitic carbonates and C-isotopically light organic matter (with roughly 25-30‰ difference between 13Ccarb and 13Creduced). The uniformitarian interpretation of such rocks is that they are powerfully indicative of a global system in which oxygenic photosynthesis (Rubisco I aerobes) dominates the partitioning of carbon. However, in applying this interpretation to the Archaean, in the absence of plants, the burden of proof is higher. The Geological Evidence Sediments in the 2.7 Ga Ngezi Gp., Belingwe belt, Zimbabwe The samples used here are chosen for their excellent preservation. In comparison to other rocks of similar age, they have experienced exceptionally gentle thermal and structural 5 Age of Rubisco histories since deposition. They are from Belingwe, Zimbabwe (~2.7 – 2.9 Ga) (Martin et al., 1980; Grassineau et al., 2001, 2002, 2006; Abell et al., 1985a), Mushandike, Zimbabwe (~2.9 Ga) (Abell et al, 1985b; Moorbath et al., 1987) and from Steep Rock, Ontario, Canada (~2.9 Ga) (Wilks and Nisbet, 1985; 1988; Davis and Jackson, 1984; Stone, 2004). Parallel S isotope studies on much of this material have already been published (Grassineau et al., 2001,2002, 2006) or are in preparation. To identify autotrophic processes, we study the entire C isotopic facies: organic carbon, carbonate carbon, and sedimentological environment, placing the interpretation in the context of the depositional setting of the host rock. Sediments in the Ngezi Group in the Belingwe belt, Zimbabwe were chosen to search for Rubisco. Shallow water coastal sands, silts, shales, laminated limestones, and stromatolites occur in both the 2.7 Ga Manjeri Formation, and in the overlying ~2.65 Ga old (Bolhar et al., 2002) Cheshire Fm. (Fig 1) (Martin et al., 1980; Grassineau et al., 2002, 2006) . In the Manjeri Fm. the stromatolites are a minor part of the succession, limited to a few small reefs a few tens of metres long and a few metres thick. Some thin (few m) carbonate beds also occur. In the Cheshire Fm. in contrast, the luxuriant stromatolite reefs are hundreds of metres long and tens of metres thick, and extensive laminated carbonate beds also occur. For limestone precipitation, water pH and/or alkalinity (Grotzinger and Kasting, 1993) was likely not very greatly different from today’s ocean. There is no evidence of input of fluids with C signatures of high temperature Fischer-Tropsch processes in any of the Cheshire Fm. rocks, which are of very low metamorphic grade. Results are shown in Fig. 3. In the Manjeri Fm. stromatolites, 13Ccarb is ~ 0‰ and 13Creduced ~ –23‰ , ranging to circa –35 ‰ or lighter in shales (Fig. 3). In the Cheshire Fm., stromatolite reefs are superbly preserved in several locations as thick reefs. The rocks are unstrained and metamorphic grade is very low (Martin et al., 1980). In these, 13Ccarb values are +0.2±0.3‰ and 13Creduced –28.6±3.3‰ (Fig. 3). In shales of the Cheshire Fm., 13Creduced ranges from –45‰ to –32‰ (Grassineau et al., 2002, 2006). 13Creduced values lighter than – 40‰. Similarly 'light' carbon isotope values also occur in the Manjeri Fm. shales. In addition, a significant subpopulation of 13Creduced ranges between –15‰ to –10‰ (Grassineau et al., 2002, 2006). In the shales, authigenic carbonate (formed within the mud) has 13Ccarb around -15 to –5‰. Figures 1 and 2 about here 2.9 Ga Mushandike (Zimbabwe) and Steep Rock (Canada) successions The oldest large-scale extant still-carbonate reefs in the geological record are at Steep Rock NW Ontario, Canada (Wilks and Nisbet, 1985, 1988) (Fig. 2), Mushandike, Zimbabwe (Abell et al., 1985b) , both sampled in this study, and in >2.84 Ga strata of the Pongola belt, South Africa (von Brunn and Hobday, 1976,Eglington et al., 2003, Gutzmer et al., 1999), not analysed in this work. Although there is substantial evidence for localised deposition of earlier (pre-3 Ga) sedimentary carbonates, they are typically silicified or otherwise replaced. Unsilicified shallow-water carbonates are largely absent in the pre-3 Ga geological record, although pre-3Ga carbonate does occur in hydrothermal settings (Nakamura and Kato, 2004). 6 Age of Rubisco Mushandike succession The Mushandike stromatolites are at least 2.83 Ga old (Moorbath et al., 1987), and were probably laid down ~2.9 Ga ago. The Mushandike calcites have undergone metamorphism and recrystallisation at around 150oC, which may have shifted 13Ccarb by 1 to 2 ‰ to heavier (more positive) ratios (Abell et al., 1985b). The Mushandike stromatolites are geographically not far from the Belingwe belt and may be roughly contemporary with the lower, ~2.9 Ga Mtshingwe Group succession in the Belingwe belt , which contains well-developed boulder beds and diamictites (Nisbet et al., 1993). Isotopic results from hand-held drillcores into the Mushandike carbonates (Fig. 3) are from our work with the late Paul Abell (Abell et al., 1985b). They show that 13Ccarb ranges from 0.1 to +1.25‰, implying that original values were similar to those in the Cheshire stromatolites (Abell et al., 1985b). Steep Rock succession The Steep Rock succession includes a reef of widely varied stromatolitic limestones and dolomites (Fig. 2) (Wilks and Nisbet, 1985, 1988) which rests unconformably on 3 Ga basement. The carbonate reef is several kilometres long and in places up to several hundred metres thick. Coincidentally with the Mushandike stromatolites, and the Pongola sequence, the Steep Rock stromatolites are also at least 2.83 Ga old (Davis and Jackson, 1985; Stone, 2004). They were subject to low-grade metamorphism at 2.55 Ga (M. Regelous unpubl. pers. comm. to EGN). 13Ccarb was likely slightly shifted in this event, as in the Mushandike rocks. The Steep Rock stromatolites are overlain by iron ores of a unit known as the “Manganiferous Paint Rock” (Wilks and Nisbet, 1988). The Paint Rock is typically about 4 wt% Mn (but locally up to 19 wt% Mn). However, the present oxidation state of the Paint Rock rock is post-depositional and probably records Cenozoic alteration (Wilks and Nisbet, 1988). Isotopic results from the Steep Rock samples collected by EGN and the late Paul Abell are shown in Figure 3. Organic carbon has 13Creduced averaging –25.4‰, varying from – 30.6 to –21.6‰. Carbonate is slightly heavier than 0‰, with 13Ccarb ranging from +0.1 to +2.9‰. Pongola Succession Eglington et al. (2003) and Veizer et al. (1990) both report carbon isotopes on carbonate samples (aragonite, calcite, ankerite, and dolomite) from the Pongola Supergroup, which is >2.84 Ga old (Gutzmer et al., 1999). These rocks have been subject to some postdepositional decarbonation. In five samples studied by Eglington et al. (2003) 13Ccarb ranges from +1.2 to +2.1‰. They infer a best estimate of 13Ccarb about 1‰ for marine carbonate at the time of deposition. Ono et al. (2005) studied organic matter in the Mozaan group and found two populations with 13Creduced of –26‰ and –32‰. As in 7 Age of Rubisco Steep Rock, some strata in the succession decribed by Eglington et al. (2003) are Mnrich. Evidence for glaciation in the Pongola rocks is discussed below. Figure 3 about here TABLE 1 Age of strata >2.83 Ga 13Ccarbonate 13Creduced Interpretation +0.1 to +2.9‰ -30.6 to –21.6‰ >2.83 Ga +0.1 to+1.25‰ Manjeri Fm. stromatolites and shales 2.7 Ga ~ 0‰ ~ -23‰ Rubisco I+II aerobes Carbonate suggests Rubisco I Probably Rubisco aerobes Cheshire Fm stromatolites Cheshire shales Pongola Supergroup (Eglington et al., 2003; not analysed in this work) 2.65 Ga +0.2±0.3‰ -28.6±3.3‰ Rubisco I -15‰ to –5‰ Range +1.2 to +2.1‰, best estimate of original value +1‰ -45‰ to –32‰ Two populations: -26‰ and -32‰ (Ono et al. 2005) Rubisco III Rubisco I and II aerobes Geological unit Steep Rock stromatolites Mushandike stromatolites >2.84 Ga Interpretation of the geological evidence: dating the origin of oxygenesis Ngezi Group: Manjeri and Cheshire Fms.: carbon isotopes The simplest explanation for the ~2.7 Ga Ngesi Group C isotopic results is that carbon was captured by photosynthesis, and then recycled by methanogens. This hypothesis is supported by several distinct lines of evidence. 1. Much of the carbon has 13Creduced in the range –30‰ to –20‰. This is the range likely to be produced by autotrophic capture by photosynthetic organisms using either Rubisco I or Rubisco II. Rubisco I (cyanobacteria) and Rubisco II (purple bacteria) organisms live in aerobic or microaerobic conditions. Hence the conditions were not anaerobic. 2. There is a distinct reduced facies, containing sulphides and clumps of organic carbon, that has 13Creduced in the range –40‰ to –30‰. This is most likely the signature of anaerobic methanogens. 8 Age of Rubisco 3. In the stromatolites, there is a bimodal distribution of carbon, with 13Ccarbonate around 0‰ and reduced carbon in the range -30 to -20‰. The 13C contrast between carbonate and organic matter is most simply interpreted as a record of carbon isotope selection by aerobic organisms, both cyanobacteria and/or aerobic purple bacteria. If it is assumed that CO2 degassed from volcanoes was similar to today, and 13C in CO2 in ambient seawater was around –5 to –10‰, then the 13Ccarb isotopic record is consistent with capture of say 20% of dissolved carbon in the water by aerobes. The finding of coexisting 13Ccarb ~0 ‰ and 13Creduced ~-30 to-20‰ in most probably interpreted as evidence for carbon capture by Rubisco I cyanobacteria. These likely lived in association with anoxygenic Rubisco II purple bacteria in the aerobic part of microbial mats. Deeper within microbial consortia, in the anoxic bases of microbial mats, the very 'light' 13Creduced suggests methanogens were probably also present and thus possibly Rubisco III organisms. Some mainly anoxic muddy settings were present nearby, just as in lagoons today anoxic mud occurs close to vigorous oxygenesis. In the shales of the Cheshire Fm., the most likely explanation of the very depleted 13Creduced (lighter than –40‰) is that carbon cycling occurred via methanogeny (Hayes, 1994) , implying Rubisco III methanogens were present in the mud, which was rich in organic-debris. It is possible that the isotopically light authigenic carbonate in the Cheshire shales formed from CO2 produced within the sediment by oxidation of methane as it rose in the mud i.e. methanotrophic precipitation. The size and purity of the reefs suggests they were probably highly productive. In the Cheshire stromatolite reefs the absence of significant clastic content implies that reefforming took place quickly, so that clastic input such as dust from unvegetated land or mud from floods was only a small part of the record. This fast growth is also supportive of the hypothesis that oxygenic photosynthesis was occurring, as only this process could have sustained the productivity needed (Kharecha et al., 2005). Independent evidence for oxygenesis in the ~ 2.7Ga Ngezi Group rocks The inference of oxygen-rich conditions is powerfully supported by independent geochemical evidence that the conditions were oxic (Siebert et al., 2005). Mo concentrations in the Belingwe sediments vary but range up to 6 ppm. In the same material, 98/95Mo values are between –2.1‰ and –1.4‰. These values, more typical of Proterozoic – modern than Archaean, indicates that Mo was present in solution. The presence of oxic water is typically necessary for soluble Mo. This in turn strongly implies that the air was sufficiently oxidising to mobilize Mo during weathering (Siebert et al., 2005) and that water conditions were oxidising (at least locally in Belingwe lagoons). There is a caveat to this: the evidence is not absolute as there are other ways to mobilize Mo: e.g., thiosulfate, which is plausible in Archean oceans, will mobilize reduced Mo. However, Fe isotope evidence also supports the presence of oxygen-rich surface waters in the Ngezi Gp. Rocks (Archer and Vance, 2006). Taken collectively, the case for oxic water becomes very strong. 9 Age of Rubisco The Cheshire Fm stromatolites are similar in age to the Carawine Dolomite in Australia. In these ~2.6 Ga rocks, Ono et al. (2003) found a small 33S signal, ranging from –2.5‰ to –1.1‰ , not inconsistent with some oxygen emission to air at this time. In contrast, the much smaller, less abundant Manjeri Fm. stromatolites, may be of similar age to the Jeerinah Fm. reported by Ono et al., (2003), in which 33S ranges from –0.1 ‰ to +8.1‰, suggesting oscillation between oxic and anoxic conditions. However, any interpretation of the 33S signal comes with a caveat comparable to that accompanying the Mo evidence. This was a time of major global volcanic activity, attested by the thick sequences of lavas worldwide (including those in the Ngezi Group). In the modern Earth, despite 21% O2 in the air, MIF is observed in volcanic ash deposited in the polar ice (Baroni et al., 2006; Savarino et al., 2003). It is thus possible that the small MIF signature seen in 2.6 to 2.7 Ga strata similarly records volcanic sulphide deposited after massive volcanic eruptions that put a global S load into the stratosphere. The small contemporary 33S signal may not record atmospheric oscillation between oxic and anoxic conditions: instead it may simply be a record of massive volcanic events that pierced the stratosphere of a uniformly oxic atmosphere. Grassineau et al., (2002, 2006, unpublished) found very wide ranges in 34S in single samples in both the Manjeri and Cheshire Fms. In one Manjeri Fm drillcore sample from relatively shallow facies, 34S ranged within a few cm from –18.3‰ to –3‰, while in a deep water sapropel sample, in a highly anaerobic facies from the same formation, 34S ranged from +7.4 to +14.3 within millimetres. Overall in the Ngezi Group, 34S ranges by 40‰, from –24‰ to +17‰ (Grassineau et al, 2002, 2006). Part of this 34S signal may come from MIF processes, via disproportionation of S8 in water. Applying Ono et al's. (2003) formulation (33S – 0.51)/0.64 ~ 34S to the 33S range from the Carawine Dolomite gives a range of MIF factors for 34S of about between about 4‰ and 1‰, small in relation to the 40‰ range observed. Thus the bulk of the 34S signal is likely to be from microbial processes, a view also expressed by Ono et al.(2003) for their samples. The large 34S signal may record biological sulphureta cycling in shallow water. Grassineau (2002,2006, unpublished) inferred the presence in shallow water of aerobic purple sulphur bacteria (Rubisco II). In stringers of highly anoxic facies, sulphides associated with carbon clumps in shallow anoxic facies may record recycling by anoxygenic photosynthesis (e.g. anaerobic green S bacteria using Rubisco-like-protein IV). Reduction of S species must have been widespread in both shallow and deep water facies, especially in highly anoxic deeper water facies in close proximity to highly depleted carbon clumps. The highly positive 34S in sulphides in deep water anoxic facies may record anaerobic methane oxidation (Rubisco III) occurring against oxidation power provided by fluids fluxing from underlying iron-containing clastics, as in the modern Black Sea (Jorgensen et al., (2004). 10 Age of Rubisco Thus the uniformitarian interpretation of the Ngezi Group sedimentary facies and C isotopic signatures (Fig. 3), with their markedly distinct organic and carbonate C signatures, and abundant other evidence of aerobic conditions, is that they are a record of ~2.7 Ga microbial consortia founded on oxygenic Rubisco I productivity. Possible evidence for oxygenesis in the ~ 2.9 Ga samples The Steep Rock, Mushandike and Pongola stromatolites, all coincidentally dated as >2.83 or >2.84 Ga, are among Earth’s oldest large-scale carbonate reefs. In all three, the carbonates may have been subject to a slight post-depositional metamorphic resetting (Abell et al., 1985b; Eglington et al., 2003) shifting 13C by a few ‰. In the three successions, results show that 13Creduced is around –25‰ , and 13Ccarb around +1 to +2‰. As with the younger Ngezi Group samples, the results, together with the characteristic difference between 13C in organic matter and carbonate suggests, though does not prove, that processes controlling carbon isotope partitioning were aerobic. The carbon isotope evidence (Fig. 3; Table 1) and the similarity of the sedimentary facies to the Belingwe material imply that if oxygenesis is accepted for the Cheshire reef, then the same uniformitarian criteria imply that the Steep Rock and Mushandike reefs were built by organisms that used Rubisco I – in other words, an oxygenic ecology based on cyanobacteria.. Supporting evidence for ~2.9 Ga oxygenesis: a) glaciation and b) 33S MIF record Direct supporting isotopic evidence for oxic geochmistry in the ~2.9 Ga rocks is not available. However, though the Steep Rock Mn-rich deposits may be reworked in the Phanerozoic, original Mn deposition was probably in the Archaean, consistent with local presence of oxygenated seawater (Kharecha et al., 2005). The hypothesis of oxygenic photosynthesis at >2.83 Ga is consistent with the kilometre-scale of the Steep Rock carbonate reef, hundreds of metres thick, which is poor in clastic material despite resting unconformably on granitic craton (Wilks and Nisbet 1985, 1988) and thus deposited proximally to a dusty landmass. The scale, thickness and facies of the reef imply a highly productive ecology, unless the deposition period was very prolonged and winds soft. There is good evidence for ~2.9 Ga glaciation. This may have bearing on the oxygen debate. In the ~2.9 Ga Mozaan Group, the upper part of the Pongola Supergroup, South Africa, Young et al. (1998) reported diamictites, with convincing evidence for glaciation. Diamictites of possible glacial origin are also found in the 2.9 Ga Witwatersrand succession (Harland, 1981). In the Belingwe belt, the ~2.9 Ga Mtshingwe Gp contains very extensive coarse diamictites and a large boulder bed (Nisbet et al., 1993). These are of uncertain origin either coastal in a region of active tectonics, or glacial, or both (Nisbet et al., 1993),. Although not our preferred hypothesis, these Mtshwinge Gp. diamictites may indeed record glacial environments. This evidence for glaciation in ~2.9Ga rocks is 11 Age of Rubisco consistent with the hypothesis that oxygenic photosynthesis began then. When oxygenic cyanobacteria first evolved, the release of free oxygen would have immediately reduced the atmospheric methane inventory as it was oxidised to CO2 . Methane is a potent greenhouse gas. Destruction of atmospheric methane by newly-released oxygen would have reduced greenhouse warming (Kharecha et al., 2005), inducing climate cooling. Moreover, in Pongola sulphides 33S is near 0‰, suggesting weak mass-independent fractionation of S isotopes (Ono et al., 2006). The MIF record from the Mozaan group supports the notion that oxygen was being produced. The 33S values of nine samples range from –0.49‰ to +0.36‰ (Ono et al., 2006). This range is much smaller than measured in much of the other Archaean record, though it is outside the narrow Phanerozoic and modern range. Ono et al. concluded that the atmosphere above the Mozaan Group sediments as they were laid down was slightly oxidised, wth oxygen levels above 10-5 but below 10-2 of present atmospheric level. From this evidence Ono et al. (2006) inferred the existence of oxygenic photosynthesis at 2.9 Ga. Siebert et al. (2005), in a ~2.95 Ga sample from the West Rand Gp. of the Witwatersrand, also find "a hint of an incipient, possibly transient rise in oxygen levels." The evidence for ~2.9 Ga glaciation does thus suggest that the global greenhouse was challenged at this time. The ~2.9 Ga MIF data imply that the challenge came from oxygen. Do the Steep Rock, Mushandike and Pongola limestones record the first appearance of Rubisco I? Absence of evidence for carbonates in older (pre 2.9 Ga) rocks Though stromatolites older than 3 Ga do occur, carbonates are typically silicified. Limestones are now absent though some deposits, now dolomitised, may originally have been aragonitic. In contrast to the kilometre-scale Steep Rock reef, prior to 2.9 Ga, very few older carbonates occur in the record, and those that do are on an extremely smallscale. The carbonates in the 3.45 Ga Strelley Pool Chert, Pilbara, Australia are controversial and may reflect special local hydrothermal settings. It is however likely that the stromatolites are indeed biogenic (see van Kranendonk et al., 2003 and references cited therein for discussion of this point), but that they formed in anoxic, not oxic, seawater. Silicified carbonates deposited between 3.4 to 3.2 Ga ago also occur in the Barberton belt (Toulkerides et al., 1998; see also Westall et al., 2006). The absence of pre-2.9 Ga limestones is consistent with evidence of high CO2 partial pressure (Hessler et al., 2004) and global atmospheric anoxia (Farquhar and Wing, 2003). Before oxygenic photosynthesis, seawater pH may have been so low that large-scale calcite precipitation was inhibited, except in rare locations of unusual alkalinity and high pH (Grotzinger and Kasting, 1993) (e.g. near hydothermal systems). 12 Age of Rubisco The post-2.9 Ga geological record preserves abundant reef carbonates. That there is no record of large reefs of limestone before ~2.9 Ga ago may simply be an accident of preservation:- that rocks formed in appropriate shallow-water settings, protected from clastic debris, have not survived. However, although the record is sparse, there are shallow-water sequences in Isua, the Pilbara and Barberton where the clastic influx was limited. Yet despite this, large carbonate reefs older than ~2.9 Ga are puzzlingly absent. Significance of the ~2.9 Ga carbonates At ~2.9 Ga ago (more precisely, >2.83 Ga ago: the rounding of 2.9 Ga is used here for convenience), the first known large-scale carbonate reef formed at Steep Rock, and similar rocks occur in the Mushandike and Pongola bodies. In each of these there is C isotope evidence most easily (though not uniquely) interpreted by the hypothesis that Rubisco I was active. Moreover, there is also evidence of glaciation, and of 33S close to 0‰ in the Pongola sequence. Prior to the evolution of Rubisco I and oxygenic photosynthesis, anoxic liquid oceans existed (van Kranendonk et al., 2003; Hessler et al., 2004). These oceans were probably sustained as liquids by a greenhouse with both high methane and high CO2 inventories under a faint young sun (Kasting and Ono, 2006; Kasting 2001). The simplest explanation of the sudden appearance of carbonates is draw-down of atmospheric CO2. If cyanobacteria capable of oxygenic photosynthesis (i.e. Rubisco I) first appeared at this time (presumably evolving from a prior anoxygenic biota), the immediate productivity burst (Kharecha et al., 2005) would take up atmospheric CO2 and hence make the oceans less acid, with rising pH. From this date, because of the prior selection of 12C by Rubisco I organisms, the remaining CO2 destined for inorganic precipitation would be correspondingly enriched in 13C. Thus the first appearance of the twinned characteristic isotopic signatures of organic carbon and of the residual carbon in sedimentary carbonates drawn from the atmosphere/ocean inventory (organic matter with 13Creduced around –20 to -30‰, in association with contemporary 13Ccarb around 0‰) is consistent with the evolution of cyanobacteria at this date. In the late Archaean the fractions of degassed carbon that entered each sink are not well known and remain controversial. For the fraction captured by organisms Bjerrum and Canfield (2004) infer 0-10%, while others (e.g. Kharecha et al, 2005) prefer values around 20%. In detail, with the presence of highly productive oxygenic photosynthesers (Schidlowski, 1988) (e.g. cyanobacterial mats in a lagoon), ambient CO2 in water would be locally depleted. This would create a local environment of rising pH in local shallow waters, permitting precipitation of carbonate reefs with 13Ccarb 0‰, as oxygen was released. The Steep Rock/Mushandike/Pongola evidence is most simply interpreted as the record of highly productive oxygenic photosynthesis that permitted the onset of carbonate deposition in local reefs. The high Mn content of some of the associated sediments lends 13 Age of Rubisco weak circumstantial support to the hypothesis that oxygenic photosynthesis began with Mn-bicarbonate solutions (Dismukes et al., 2001). However, most modern cyanobacterial cells are picoplankton. If oxygenic cyanobacteria existed in reef mats, then it is highly likely picoplankton had also evolved. Given that CO2 in shallow water equilibrates with ambient air (especially if carbon anhydrase had already evolved), and air is well-mixed globally, then the large Steep Rock reef with 13Ccarb of 0‰ further suggests global draw-down of CO2 (as distinct from transient depletion of CO2 dissolved in lagoonal water) began at this time. Global CO2 drawdown would be capable of reducing greenhouse warming enough to initiate glaciation and hence in turn to inhibit the cyanobacterial bloom and slow further CO2 capture. The global environment 2.9 Ga ago. Unless glacial conditions developed, newly-evolved oxygen production by cyanobacterial plankton would be unlikely to overwhelm the large inventory of reduction power in anoxic deeper water. The parallel of the modern Black Sea shows that enhanced surface productivity produces more reduced debris to sink to the bottom: the bottom becomes more, not less anoxic. Only cold glacial conditions or salt-enrichment produce dense sinking oxygenated waters. Thus, after the evolution of oxygenesis, most of the oxygen would likely be in a specific depth range in the photic zone water, especially in coastal lagoons where it would be protected from upwelling anoxic bottom water. As time continued, oxygen build-up in the air would only occur if fresh volcanic emission of CO2 permitted photosynthesis and O2 release that was markedly more rapid than the consequent competing emission of equal amounts of reduction power via methane from decaying organic matter created by the new biological productivity. Oxygen would thus become a transient photic zone gas in proximity to anoxia (as, reversely, Black Sea methane is today), not a major component of the air. At 2.9 Ga, the pre-existing global atmospheric CO2 inventory (perhaps around 1000ppm (Kasting and Ono in press) would have set a limit on total global oxygen release by a bloom of newly-evolved cyanobacteria. Thus build-up of free atmospheric oxygen may have been limited. Capture of most of the prior CO2 inventory with release of oxygen to air would have created atmospheric oxygen only to a thousandth of a bar. More likely the new atmospheric oxygen inventory was less than this limit: the oxygen would have been released into water, where much would have gone into reaction with reduced anoxic deeper water or reduced minerals. In the biologically active photic zone this would have created an oxygenated water body under a reducing sky. There is a reverse modern parallel for this in the Black Sea (Euxine sea). Here the deeper water below about 50m is strongly anoxic (euxinic), even though it is overlain by strongly oxic waters and these in turn by the modern oxic atmosphere. Oxygenesis may also have been self-limiting. Rubisco I carries with the capacity for forcing glacial/interglacial cycles.. Its specificity for CO2 increases as temperature drops towards freezing (Tcherkez et al., 2006). The possible sequence is as follows: 1) Release of oxygen even in trace quantities would increase the oxidative power of the atmosphere. This would proportionately remove or reduce atmospheric methane, cutting the 14 Age of Rubisco greenhouse warming that sustained global temperatures. 2) In the colder temperatures, Rubisco's increased specificity (Tcherkez et al.,2006) would initially exacerbate the effect, but removing CO2 as well as methane. 3) Glaciation would result, eventually inhibiting further oxygen production. 5) Eventually warmth would be restored by emission of methane from deep stores of organic debris on the continental edge and proximal oceanic shelves, newly-enriched in organic matter by the earlier productive microbial ecology. Perhaps after an initial outburst of productivity at 2.9 Ga that partially drew down a preexisting inventory of ~1000 ppm CO2 and released matching oxygen, a balance was attained between methane return from sediments and temperature-restricted oxygenic photosynthesis, a view supported by the record of mass independent fractionation of sulphur isotopes (Farquhar and Wing, 2003; Kasting and Ono, in press). Rubisco Compensation and atmospheric CO2 : O2 mixing ratios “It is clear that there will be a limiting minimum concentration of carbon dioxide, below which it is impossible for vegetation to live” (Macgregor, 1927). If CO2 is too low, life cannot extract it from the air or from dissolved oceanic sources. Conversely, if O2 is too high the living organism self-oxidises and instead of extracting CO2 from the air, it gives up carbon to the air, returning CO2. If unduly prolonged, this is fatal to the cell. The balance between O2 emission and CO2 uptake by cells carrying out oxygenic photosynthesis is linked to Rubisco I specificity for carbon over oxygen. Carbon capture and release by Rubisco I and II are subject to ‘compensation’ controls (Tolbert, 1994; Berry et al., 1994; Tolbert et al., 1995). More generally, Rubisco’s compensation controls define a limiting field for the permissable relative burdens of CO2 and O2 in the air if oxygenic photosynthesis is to occur. In general, discussion of CO2:O2 ratios in the geological literature focusses on controls by inorganic chemistry rather than biochemistry. However, if Rubisco-mediated oxygenesis did indeed exist in the period between ~2.9 Ga and ~2.4 Ga ago, as the isotopic evidence above implies, then these oxygenic organisms would have been subject to Rubisco compensation controls. If so, the compensation limits on atmospheric composition are worth investigating. Figure 4 about here Rubisco's CO2 and O2 compensation points The CO2 compensation point (CO2 at any given O2 level is the CO2 concentration at which net CO2 fixation is zero. CO2 varies linearly with ambient O2. Below CO2 there is net CO2 loss from photorespiration. As an illustration Tolbert et al. (1995) studied chloroplasts in tobacco seedlings (Fig. 4). - note these trends are only illustrative, and are likely to be temperature and pressure dependent). 15 Age of Rubisco For each Rubisco-dependent aerobic autotroph, specific CO2 and O2 lines set permissive habitat limits for dioxygen and CO2. Collectively, compensation is set by the broad community of photosynthesing organisms, only surviving if dioxygen concentrations are below communally set O2 and CO2 is above communally set CO2 . There is striking disproportion: in the permissive zone, O2 is in per cent, but CO2 in parts per million, a difference of three orders of magnitude. Within the permissive zone, evolution by natural selection will tend to maximise productivity and hence take up carbon, driving the CO2 mixing ratio downwards towards the CO2 line. The modern atmospheric disproportion between CO2 tends towards the O2 CO2 intersect. O2, with an atmospheric lifetime of ~107 years, may be linked to average Quaternary/lateTertiary CO2 (Tolbert et al., 1995), falling in glacial times below 200 ppm CO2, at 21% O2. Because of the difference in size of the reservoirs, CO2 will respond quickly to any environmental change, on a timescale of a few centuries, but the dioxygen burden of the air will shift more slowly, over millions of years. On the modern planet, glacial climate dominates. In engineering terms this is a ‘ramp’ type of control system. The huge O2 reservoir in the modern air imposes long-term stability on CO2. Volcanic injections of CO2 make very little impact on oxygen. For example, doubling CO2 would induce rapid photosynthetic release of dioxygen, barely shifting the oxygen reservoir. The system will regress again to the CO2 line. Compensation and Rubisco specificity in the Late Archaean The CO2 line suggests that Archaean cyanobacteria could operate at very low CO2 mixing ratios, with very low ambient O2, provided the climate was kept warm enough by another greenhouse gas such as methane. For example in modern tobacco seedlings, which are analogous to exposed chloroplasts (i.e. cyanobacteria), for growth at O2 concentrations as low as 2%, the CO2 mixing ratio must be above 9 ppm (Tolbert et al., 1995). Lower O2 mixing ratios would permit lower ambient CO2 mixing ratios. Such conditions could possibly be attained in water in so-called oxygen oases. It is possible to imagine an anoxic atmosphere with very low free O2 mixing ratios, but with higher O2 in photic zone waters. In lagoons of active stromatolite growth conditions may have been aerobic, with O2 substantially higher than in the air above. The compensation constraints suggest a possible Late Archaean ecology in which the atmosphere contained a burden of 10-100 ppm of CO2. If so, the crucial atmospheric greenhouse gas would have been the burden of methane, perhaps in similar mixing ratios and hence a much longer lifetime than today, supported by strong emission from organicrich sediments and overturn of anoxic deeper waters. In this setting, free oxygen would compete with methane as a trace component of the air, but in shallow waters could build towards percent levels late in the afternoon. Productive cyanobacteria would sustain an ecology that supported abundant methanogens and consequent methane emission. This in turn would provide substrate for methanotrophs, which would also take up excess oxidant. Aerobic water may also have existed a few metres below the surface of the open 16 Age of Rubisco ocean, maintained by picoplanktonic cyanobacteria at tightly controlled preferred photosynthetic depths. The main danger to such an ecology would be over-exuberant emission of oxygen, to a level that threatened the methane greenhouse and induced glaciation (see discussion above). This, however, would normally be self-correcting to maintain anoxia, as glaciation would suppress oxygenesis in shallow waters while methane emission from sediment-hosted methanogens would continue. Only catastrophic failure of this mechanism could tip the system to a permanent snowball. That would allow CO2 to build-up, and the nutrient load in the oceans, and hence, when the glaciation ended, would provide a massive substrate for oxygenesis. Rubisco thus permits two stable states of the air (Nisbet 2002). Either can sustain liquid water on the ocean surface: one anoxic with methane as the greenhouse gas, and the other oxic, with CO2 as the main greenhouse gas. Goldblatt et al., (2006) showed that a bistable atmosphere is also imposed by ozone chemistry. Compensation in snowball events and after In the sustained global ‘snowball’ around 2.3 Ga ago (Kopp et al., 2005), catastrophic failure did indeed occur. Photosynthetic productivity must have been very low for millions of years. This would have permitted volcanic CO2 to accumulate to ~12% of the air (Caldeira and Kasting, 1992) before the snowball broke down. After snowball collapse, with very high atmospheric CO2, acidophile cyanobacteria would have bloomed in the newly warmed degassing oceans, which would be anoxic, rich in nutrients such as dissolved iron, and acid compared to today. The bloom of oxygenic photosynthesisers would rapidly extract carbon from the huge inventory. Conversion of a significant part of the (say) 10-12% CO2 inventory to dioxygen would have driven the O2:CO2 ratio far up the CO2 line until it was checked either by using up a key prerequisite (e.g. available photic zone Fe), or by consuming the CO2 inventory, or by reaching the O2 line. The compensation control in turn permitted sharply increased oxygen when the climate suddenly warmed. This would produce a stable oxygen-rich system, with greenhouse warming supported by CO2 alone, little aided by methane. The post-snowball rise in oxygen (Fig. 5) thus is consequential on the CO2 increase during the snowball, when rubisco compensation failed as oxygenic photosynthetic life retreated to a few liquid oases and CO2 was able to increase unchecked. The stability of non-glaciated intermediate states, as in the Proterozoic, would depend on high methane emission rates and relatively long atmospheric lifetimes for methane (i.e. cool dry air, with low OH formation: such conditions occur when oceans are cool and continents dry). The snowballs in the late Proterozoic may have been the final increment, the CO2 buildup in the snowballs permitting post-snowball compensation with high O2 and high CO2 and the stable attainment of a warm post-snowball Earth, close to the limiting O2 barrier. 17 Age of Rubisco Subject to rubisco and greenhouse-controls, the global environment may have two stable end states – one low on the CO2 line with very little O2 except in oases of oxygenated water, low CO2 and significant methane, and a second state with high O2, no atmospheric methane, and several hundred ppm CO2, close to the upper bound of the CO2 line where it intersects the O2 line. During periods of general global warmth, atmospheric CO2 mixing ratio is likely to be high. Organisms whose Rubisco has high CO2 specificity only have this property at low temperatures: at higher temperatures the specificity drops (Tcherkez et al., 2006). Thus in warm high CO2 times, community optimisation by selective competition would, within the global population of organisms, broadly tend to favour those with low CO2 specificity and high productivity. Consequently, isotopic fractionation into organic matter would be reduced in warmer periods, but total production and burial of organic carbon would increase. In the balancing carbonate reservoir, 13Ccarb may thus change little despite the fluctuation in productivity. Anoxygenic photosynthesis is very old, dating back to the early Archaean (Tice and Lowe, 2004, Grassineau et al., 2006a, Westall et al., 2006). The evidence discussed here implies that specifically oxygenic (Rubisco I) photosynthesis may have begun ~2.9 Ga ago. We argue that since then, the balance between the carboxylase and oxygenase roles of Rubisco I: that Rubisco I is the chief architect of the atmosphere. The role of nitrogenase, as a partner to Rubisco, will be discussed by us elsewhere. Rubisco’s specificity (its supposed ‘inefficiency’), has controlled atmospheric CO2 and the greenhouse, sustaining the disproportion between dioxygen and CO2 in the air. In the emergence from the snowball catastrophes, after build-up of a very CO2 rich atmosphere, that control stabilised the present aerobic ecology and made possible the evolution of metazoa. Figure 5 about here Analytical Methods 13Cred was analysed on a VG/Fisons/Micromass ‘Isochrom-EA’ system, consisting of an elemental analyser (EA1500 Series 2) on line to an Optima mass spectrometer operating in He continuous flow mode (Grassineau et al., 2006b). Precision better than ±0.1‰ was obtained on hand-picked samples of 0.07 mg for pure carbon, to 30 mg for whole rock with 0.1wt%C. Standards include NBS 21 and IAEA-CO9. Blank contamination from tin capsules has been measured at <34ppmC in the RHUL laboratory. Pure carbonates (0.5mg) were measured for 13Ccarb using an Isocarb automated carousel connected to a PRISM mass spectrometer. Impure carbonates were analysed using a modified Micromass Multiflow connected to an Isoprime mass spectrometer. Internal precision is better than ±0.07‰ for 13Ccarb and ±0.10‰ for 18O for both systems. Standards are NBS 19 limestone and a laboratory calcite. Acknowledgements To be added if the paper is accepted for publication after anonymous review References 18 Age of Rubisco Abell, P.I., McClory, J. Martin, A. and Nisbet, E.G. (1985a). Archean stromatolites from the Ngesi Group, Belingwe Greenstone Belt, Zimbabwe. Preservation and stable isotopes - preliminary results. Precambrian Research, 27, 357-383. Abell, P.I., McClory, J., Martin, A., Nisbet, E.G. and Kyser, T.K. (1985b). Petrography and stable isotope ratios from Archean stromatolites, Mushandike Fm., Zimbabwe. Precambrian Research, 27, 385-398. Allwood, A.C., Walter, M.R., Kamber, B.S., Marshall, C.P. and Burch, I.W. (2006) Stromatolite reef from the Early Archaean era of Australia. Nature 441, 714-718. Archer, C., and Vance, D., (2006) Coupled Fe and S isotope evidence for Archean microbial Fe(III) and sulfate reduction. Geology, 34, 153 Ashida, H., Saito, Y., Kojima, C., Kobayashi, K., Ogasawara, N., and Yokota, A. (2003) A functional link between Rubisco-like protein of Bacillus and photosynthetic Rubisco. Science, 302, 286-290. Ashida, H., Danchin, A., Yokota, A., (2005) Was photosynthetic RuBisCO recruited by acquisitive evolution from RuBisCO-like proteins involved in sulfur metabolism? Research in Microbiology 16, 611-18. Baroni, M., Thiemens, M.H., Delmas, R.J. and Savarino, J. (2006) Mass-independent sulfur isotopic compositions in stratospheric volcanic eruptions. Science 315, 84-87. Berner, R.A., Petsch, S.T., Lake, J.A., Beerling, D.J., Popp, B.N., Lane, R.S., Laws, E.A., Wetley, M.B., Cassar, N., Woodward, F.I. and Quick, W.P. (2000 ) Isotopic fractionation and atmospheric oxygen: implications for Phanerozoic O2 evolution. Science 287, 16301633. Berry, J.A., Collatz, G.J., Guy, R.D., and Fogel, M. (1994) The compensation point: can a physiological concept be applied to global cycles of carbon and oxygen? In: Tolbert, N.E. and Preiss, J. Regulation of Atmospheric CO2 and O2 by Photosynthetic Carbon Metabolism Oxford: oxford Univ. Press, 234-248. Bjerrum, C.J. and Canfield, D.E. (2004) New insights into the burial history of organic carbon on the early Earth. Geophysics, Geosystems, 5 Q08001, doi:10.1029/2004GC000713 Bolhar, R., Hofmann, A., Woodhead, J.D., Hergt, J.M., and Dirks, P., (2002) Pb- and Ndisotope systematics of stromatolitic limestones from the 2.7 Ga Ngezi Group of the Belingwe Greenstone Belt: constraints on timing of deposition and provenance: Precambrian Research, v. 114, 3-4, p. 277-294. Brocks, J.J, Logan, G.A., Buick, R. and Summons,R.E. (1999) Archean molecular fossils and the early rise of eukaryotes. Science, 285, 1033-1036. 19 Age of Rubisco Brocks, J.J., Buick, R., Summons, R.E., and Logan, G.A. (2003) A reconstruction of Archean biological diversity based on molecular fossils from the 2.78 to 2.45 billionyear-old Mount Bruce Supergroup, Hamersley basin, Western Australia. Geochimica et Cosmochimica Acta, 67, 4321-4335 Buick, R. (1992) The antiquity of oxygenic photosynthesis: evidence from stromatolites in sulfate-deficient Archean lakes, Science , 255, 74-77. Buick, R., Dunlop, J. S. R. and Groves, D. I. (1981) Stromatolite recognition in ancient rocks: An appraisal of irregularly laminated structures in an Early Archaean chert-barite unit from North Pole, Western Australia. Alcheringa, 5, 161-181 Caldeira, K., Kasting, J.F. (1992) Susceptibility of the early earth to irreversible glaciation caused by carbon dioxide clouds. Nature 359, 226- 228. Canfield, D.E. (2005) The early history of atmospheric oxygen. Annual Review of Earth and Planetary Sciences,33, 1-36. Davis, D.W. and Jackson, M.C., (1985) Preliminary U-Pb zircon ages from the Lumby Lake – Marmion lake area, districts of Kenora and Rainy River. In: Summary of fieldwork and other activities, Wood, J., White, O.L., Barlow, R.B. and Colvine, A.C. (eds). Ontario Geological Survey, Miscellaneous Paper, 126, 791-794. Des Marais DJ, Strauss H, Summons RE, Hayes JM.(1992) Carbon isotope evidence for the stepwise oxidation of the Proterozoic environment Nature 359, 605-9. Dismukes, G.C., Klimov, V.V., Baranov, S.V., Kozlov, Y.N., DasGupta, J., and Tyryshkin, A. (2001) The origin of atmospheric oxygen on Earth: the innovation of oxygenic photosynthesis. Proc. Natl. Acad. Sci. U.S.A, 98, 2170- 2175. Eglington, B.M., Talma, A.S., Marais, S., Matthews, P.E., and Dixon, J.G.P. (2003) Isotopic composition of Pongola Supergroup limestones from the Buffalo River gorge, South Africa. South African J. Geology, 106, 1-10. Erez, J., Bouevitch, A, and Kaplan, A. (1998) Carbon isotope fractionation by photosynthetic aquatic microorganisms: experiments with Synechococcus PCC7942, and a simple carbon flux model. Canadian Journal of Botany, 76, 1109-1118. Farquhar, J., Bao, H., and Thiemans, M. (2000) Atmospheric influence of Earth’s earliest sulfur cycle. Science 289, 756-758. see also comment by Ohmoto, H., Yamaguchi, K.E. and Ono, S. (2001) Questions regarding Precambrian sulfur fractionation, and Response by Farquhar et al., Science, 292, 1959a Farquhar, J. and Wing, B.A. (2003) Multiple sulfur isotopes and the evolution of the atmosphere. Earth Planetary Science letters, 213, 1-13. 20 Age of Rubisco Goericke, R., Fry, B. (1994) Variations of marine plankton 13C with latitude, temperature, and dissolved CO2 in the world ocean. Global Biogeochemical Cycles 8, 85-90. Goldblatt, C., T. M. Lenton and A. J. Watson (2006). Bistability of atmospheric oxygen and the great oxidation. Nature 443: 683-686. Grassineau, N.V., (2006) High-precision EA-IRMS analysis of S and C isotopes in geological materials. Applied Geochemistry, 21, 756-765. Grassineau, N.V., Nisbet, E.G. , Bickle, M.J. Fowler, C.M.R., Lowry, D., Mattey, D.P., Abell, P. and Martin, A. (2001) Antiquity of the biological sulphur cycle: evidence from sulphur and carbon isotopes in 2700 million year old rocks of the Belingwe belt, Zimbabwe. Proc. R. Soc. Lond., B268, 113-9. Grassineau, N.V., Nisbet, E. G., Fowler, C.M.R., Bickle, M.J., Lowry, D., Chapman, H.J., Mattey, D.P., Abell, P., Yong, J., and Martin, A., (2002), Stable isotopes in the Archean Belingwe belt, Zimbabwe: evidence for a diverse microbial mat ecology: In: The Early Earth: Physical, Chemical and Biological Development. Fowler, CMR., Ebinger, C.J. and Hawkesworth, C.J. (Eds) Geological Society London, Special Publi., v. 199, p. 309-328. Grassineau, N.V., Abell, P., Appell, P.W.U., Lowry, D., and Nisbet, E.G. (2006) Early life signatures in sulphur and carbon isotopes from Isua, Barberton, Wabigoon (Steep Rock) and Belingwe Greenstone belts. In: Evolution of Early Earth's Atmosphere, Hydrosphere and Biosphere - Constraints from Ore Deposits. Eds: S.E. Kesler and H. Ohmoto. Geological Society of America. Special Publication 198, 33-52. Grotzinger, J.P. and Kasting, J.F. (1993) New constraints on Precambrian ocean composition. Journal of Geology, 101, 235-243. Gutzmer, J., Nhleko, N., Beukes, N.J., Pickard, A., and Barley, M.E. (1999), Geochemistry and ion microprobe (SHRIMP) age of a quartz porphyry sill in the Mozaan Group of the Pongola Supergroup; implications for the Pongola and Witwatersrand Supergroups. South African J. Geology, 102, 139-146. Guy, R.D., Fogel, M.L and Berry, J.A. (1993) Photosynthetic fractionation of the stable isotopes of oxygen and carbon. Plant . Physiol., 1010, 37-47. Hanson, T.E. and Tabita, F.R. (2001) A ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco)-like protein from Chlorobium tepidum that is involved with sulfur metabolism and the response to oxidative stress. Proc. Natl. Acad. Sci. 98, 4397-4402. 21 Age of Rubisco Harland, W.B. (1981) The late Archaean Witwatersrand conglomerates, South Africa. In: Earth's pre-Pleistocene glacial record (eds M.J. Hambrey and W.B. Harland). Cambridge; Cambridge Univ. Press. pp185-187. Hayes, J.M. (1994) Global methanotrophy at the Archean-Proterozoic transition. In: Bengtson, S., (ed.) Early life on Earth. New York: Columbia University Press. 220-236. Hayes, J.M. (2001) Fractionation of the isotopes of carbon and hydrogen in biosynthetic processes. In Valley, J.W. and Cole, D.R. (2001) Mineralogical Society of America Short Course nosams.whoi.edu/jmh/ 33p. Hayes, J.M. (2004) Isotopic order, biogeochemical processes, and earth history. Geochimica Cosmochimica Acta, 68, 1691-1700. Hessler, A.M., Lowe, D.R., Jones, R.L., Bird, D.K. (2004) A lower limit for atmospheric carbon dioxide levels 3.2 billion years ago. Nature, 428, 736-8. Hoffman, P.F., Kaufman, A.J., Halverson, G.P., and Schrag, D.P. (1998) A Neoproterozoic snowball Earth. Science, 281, 1342- 1346. Holland, H.D. (1999) When did the Earth's atmosphere become oxic? A reply. The Geochemical News,100, 20-22. Jahnke L.L.1; Summons R.E.; Hope J.M.; Des Marais D.J. (1999) Carbon isotopic fractionation in lipids from methanotrophic bacteria II: the effects of physiology and environmental parameters on the biosynthesis and isotopic signatures of biomarkers Correlation of the hopanoids from extant methylotrophic bacteria with their fossil analogues. Geochimica et Cosmochimica Acta, 63, 79-93. Jorgensen, B. B., Boettcher, M.E., Lueschen, H., Neretin, L.N., and Volkov, I.I. (2004) Anaerobic methane oxidation and a deep H2S Sink generate isotopically heavy sulfides in Black Sea sediments. Geochimca Cosmochimica Acta, 68, 2095-2118. Joshi, H.M. and Tabita, F.R. (1996) A global two-way component signal transduction system that integrates the control of photosynthesis, carbon dioxide assimilation and nitrogen fixation. Proceedings National Academy of Sciences (USA), 93, 14515-20. Karner, M.B., DeLong, E.F., and Karl, D.M. (2001) Archaeal dominance in the mesopleagic zone of the Pacific ocean. Nature, 409, 507-510. Kasting, J.F. (2001) The rise of atmospheric oxygen. Science 293, 819-820. Kasting, J.F., Ono., S. (2006) Paleoclimates: the first two billion years. Phil. Trans. R. Soc. B. doi: 10.1098/rstb.2006.1839 (in press) 22 Age of Rubisco Kharecha, P., Kasting, J., and Siefert, J. (2005) A coupled atmosphere-ecosystem model of the early Archean Earth. Geobiology, 3, 53-76. Kopp, R.E., Kirschvink, J.L., Hilburn, I.A., and Nash, C.Z. (2005) The Paleoproterozoic snowball Earth: a climate disaster triggered by the evolution of oxygenic photosynthesis. Proceedings. National. Academy of Sciences, 102, 11131-6. van Kranendonk, M.J., Webb, G.E. and Kamber, B.S.(2003) Geological and trace element evidence for a marine sedimentry environment of deposition and biogenicity of 3.45 Ga stromatolitic carbonates in the Pilbara craton, and support for a reducing Archaeaan ocean. Geobiology, 1, 91 Londry, K.L. and Des Marais, D.J. (2003) Stable carbon isotope fractionation by sulfatereducing bacteria. Applied and Environmental Microbiology, 69, 2942-2949. Londry , K.L., Jahnke, L.L., and Des Marias, D.J. (2004) Stable carbon isotope ratios of lipid biomarkers of sulfate-reducing bacteria. Applied and environmental microbiology, 70, 745-751. Macgregor, A.M. (1927) The problem of the Precambrian atmosphere South African Journal of Science, 24, 155-172. Martin, A., Nisbet, E. G., and Bickle, M. J., (1980), Archean Stromatolites of the Belingwe Greenstone Belt: Precambrian Research, v. 13, p. 337-362. McClory, J.P., (1988), Carbon and Oxygen Isotopic Study of Archean Stromatolites From Zimbabwe: Unpublished PhD thesis, Kingston, University of Rhode Island, 107 p. Moorbath, S.; Taylor, P. N.; Orpen, J. L.; Wilson, J. F.; Treloar, P (1987) First direct radiometric dating of Archean stromatolitic limestone Nature 326,. 865-867. Nakamura, K. Kato, Y. (2004) Carbonatization of oceanic crust by the seafloor hydrothermal activity and its significance as a CO2 sink in the Early Archean. Geochim. Cosmochim. Acta 68, 4595 – 4618.. Nisbet, E.G. (1987) The Young Earth. London: G. Allen and Unwin, 402pp. Nisbet, E.G. and Wilks, M.E. (1989). Archean stromatolite reef at Steep Rock Lake, Atikokan, Northwestern Ontario, in H. H. J. Geldsetzer, N. P. James and G. E. Tebbutt, eds, Canadian Society of Petroleum Geologists, Memoir 13, 89-92. Nisbet, E.G., M.J. Bickle, A.Martin and J.L.Orpen (1993) Sedimentology of the Brooklands Formation, Zimbabwe: Development of an Archaean greenstone belt in a rifted graben. In : Bickle, M.J. and E.G. Nisbet (1993) The Geology of the Belingwe Greenstone Belt.: A study of the evolution of Archaean continental crust. Geol. Soc. Zimbabwe Special Publication 2, A.A. Balkema, Rotterdam. 87-120. 23 Age of Rubisco Nisbet, E.G., Cann, J.R., and van Dover, C.L. (1995) Origins of photosynthesis. Nature, 373, 479-480. Nisbet, E.G. (2002) The influence of life on the face of the Earth: garnets and moving continents, Geol. Soc. London Fermor Lecture, in: Fowler, C.M.R. et al. The early Earth: physical, chemical and biological development, Geological Society London, Special Publication 199, 275-308. Ohmoto, H. (1997) When did the Earth's atmosphere become oxic?, The Geochemical News, 93,12-13. Ohmoto, H., Kakagawa, T., and Lowe, D.R. (1993) 3.4 billion year old pyrites from Barberton, South Africa: Sulfur isotope evidence. Science, 262, 555-557. Ohmoto, H., Yamaguchi, K.E. and Ono, S. (2001) Questions regarding Precambrian sulfur fractionation, and Response by Farquhar et al., Science, 292, 1959a. Ono, S., Eigenbrode, J.L., Pavlov, A.A., Kharecha, P., Rumble, D. III, Kasting, J.F., and Freeman, K.H. (2003) New insights into Archean sulfur cycle from mass-independent sulfur isotope records from the Hamersley Basin, Australia. Earth and Planetary Science Letters, 213, 15-30. Ono, S. Beukes, N. Rumble, D., and Fogel, M. (2006) Early evolution of atmospheric oxygen from multiple sulfur and carbon isotope records of the 2.9 Ga Mozaan Group of the Pongola Supergroup, Southern Africa. South African Journal of Geology in press Rosing, M.T. and Frei, R. (2004) U-rich Archaean sea-floor sediments from Greenland – indications of >3700 Ma oxygenic photosynthesis. Earth and Planetary Science Letters, 6907 1-8. Savarino, J., Romero, A., Cole-Dai, J., Bekki, S., and Thiemens. M.H. (2003) UV induced mass-independent sulfur-isotope fractionation in stratospheric volcanic sulfate. Geophysical Research Letters, 30, doi:10.1029/2003GL018134. Schidlowski, M. (1988) A 3,800 million year record of life from carbon in sedimentary rocks. Nature, 333, 313-8. Schidlowski, M. (2002) Sedimentary carbon isotope archives as recorders of early life: implications for extraterrestrial scenarios. Ch. 11 In: Fundamentals of Life, G. Palyi, C. Zucchi and L. Caglioti (eds.) Editions scientifiques et medicales. Elsevier: Amsterdam. 307-329. Schidlowski, M. and Aharon, P. (1992) Carbon cycle and carbon isotopic record: Geochemical impact of life over 3.8 Ga of Earth history in: Schidlowski., M. et al. Early 24 Age of Rubisco Organic evolution: implications for mineral and energy resources. Springer-Verlag: Berlin 147-175. Schouten, S. Strous, M., Kuypers, M.M.M., Rijpstra, W.I.C., Baas, M., Schubert, C.J., Jetten, M.S.M. and Damste, J.S.S. (2004) Stable carbon isotope fractionations associated with inorganic carbon fixation by anaerobic ammonium-oxidising bacteria. Applied and Environmental Microbiology, 70, 3785-3788. Siebert, C., Kramers, J.D., Meisel, T., Morel, P., and Nagler, T.F.(2005) PGE, Re-Os, and Mo isotope systematics in Archean and early Proterozoic sedimentary systems as proxies for redox conditions of the early Earth. Geochimica Cosmochimica Acta, 69, 1787-1801. Sleep, N.H. and Zahnle, K. (2001) Carbon dioxide cycling and implications for climate on ancient Earth. Journal of Geophysical Research, 106, 1373-1399. Stone, D., (2004) Project unit 95-014. Geology of the Lac des Mille Lacs area. in OGS Summary of Field Work and Other Activities 2004. Ontario Geological Survey Open File Report 6145, 12-1 – 12-13. Summons, R.E., Jahnke, L.L., Hope, J.M., and Logan, G.A. (1999) 2-Methylhopanoids as biomarkers for cyanobacterial oxygenic photosynthesis. Nature, 400, 554-7. Tcherkez, G.G.B., Farquhar, G.D., Andrews, T.J. (2006) Despite slow catalysis and confused substrate specificity, all ribulose bisphospahte carboxylases may be nearly perfectly optimized. Proc. Natl. Acad. Sci. 103, 7246 . Tice, M.M., and Lowe, D.R. (2004) Photosynthetic microbial mats in the 3416-Myr-old Ocean Nature 431, 549-552. Tolbert, N.E. (1994) Role of photosynthesis and photorespiration in regulating atmospheric CO2 and O2. In: Regulation of atmospheric CO2 and O2 by photosynthetic carbon metabolism. (eds.) Tolbert, N.E. and Preiss, J. Oxford: Oxford Univ. Press. 8-33. Tolbert, N.E., Benker, C., and Beck, E. (1995) The oxygen and carbon dioxide compensation points of C3 plants: possible role in regulating atmospheric oxygen. Proc. Natl. Acad. Sci. USA, 92, 11230-11233. Toulkerides, T., Goldstein, S.L., Clauer, N., Kroner, A., Todt, W., and Schidlowski, M., (1988), Sm-Nd, Rb-Sr and Pb-Pb dating of silicic carbonates from the early Archaean Barberton Greenstone Belt, South Africa: evidence for post-depositional isotopic resetting at low temperature. Precambrian Res. 92, 129-144. Van Kranendonk, M.J., Webb, G.E., and Kamber, B.S. (2003) Geological and trace element evidence for a marine sedimentary environment of deposition and biogenicity of 25 Age of Rubisco 3.45 Ga stromatolitic carbonates in the Pilbara Craton, and support for a reducing Archaean ocean. Geobiology, 1, 91-108. Veizer, J., Clayton, R.N., Hinton, R.W., Von Brunn, V., Mason, T.R., Buck, S.G. and Hoefs, J. (1990) Geochemistry of Precambrian carbonates 3: shelf seas and non-marine environments of the Archaean. Geochimica et Cosmochimica Acta, 54, 2717-2729. von Brunn, V. and Hobday, D.K. (1976) Early Precambrian tidal sedimentation in the Pongola Supergroup of South Africa. Journal of Sedimentary Petrology, 46, 670-9. Westall, F. (2005) Life on the early Earth: a sedimentary view. Science 308, 366-367. Westall, F, de Ronde, C.E.J., Southam, G, Grassineau, N, Colas, M., Cockell, C, Lammer, H., (2006). Implications of a 3.472-3.333 Ga-old subaerial microbial mat from the Barberton greenstone belt, South Africa for the UV environmental conditions on the early Earth. Phil. Trans. Roy. Soc.B, 185, 1857-1875. Wilks, M.E. and Nisbet, E.G. (1985). Archaean stromatolites from the Steep Rock Group, northeastern Ontario. Canadian Journal of Earth Sciences, 22, 792-799. Wilks, M.E. and Nisbet, E.G. (1988) Stratigraphy of the Steep Rock Group, northwestern Ontario: a major Archean unconformity and Archaean stromatolites. Canadian Journal of Earth Sciences, 25, 370-391. Young, G.M., von Brunn, V., Gold, D.J.C., and Minter, W.E.L. (1998) Earth’s oldest reported glaciation; physical and chemical evidence from the Archean Mozaan Group (~2.9 Ga) of South Africa. Journal of Geology, 106, 523-538. FIGURE CAPTIONS Figure 1. 2.65 Ga stromatolites, Cheshire Fm., Belingwe, Zimbabwe Figure 2. ~2.9 Ga, stromatolites, Steep Rock, NW Ontario, Canada Figure 3 C isotope measurements from the Steep Rock Lake stromatolites and from the Manjeri and Cheshire Fms. in the Belingwe greenstone belt. Data fromAbell et al., 1985, Grassineau et al. 2001, 2002, 2006 and NG unpublished. Organic carbon at ~-30‰ to –25‰ may show the signature of Rubisco I in aerobic conditions with abundant CO2, while 13C –45‰ to –35‰ suggests carbon processed by methanogens. The 13C 0‰ stromatolitic carbonates, reflecting atmospheric input via ocean/air CO2 exchange and extraction of a light carbon fraction, are interpreted as indirect evidence for 26 Age of Rubisco oxygenic photosynthesis. One explanation of calcite around –10‰ to –5‰ is that it derives from authigenic CO2 released by methanotrophy. Figure 4 Rubisco Compensation lines for tobacco chloroplasts (plotted from data in Tolbert et al., 1995, who worked at lab temperature and pressure). CO2 is near-horizontal line, with CO2 (ppm)= 2.13 O2 % + 3.89 O2 is steep line with O2(%) = 0.0246CO2 ppm +17.94 For net growth, atmospheric CO2 levels must be on or above the CO2 line, and O2 on or below O2 . CO2 is in parts per million while O2 is in percent. "Permitted zone" shows region in which Rubisco I is capable of sustaining oxygenic photosynthesis. In practice, in water, photic zone CO2 is quickly depleted. Resupply must come from air so, even with efficient carbonic anhydrase, there must be a gradient (i.e. the effective atmospheric CO2 line may be parallel to, but above, that shown). Triangle shows approximate composition of air in last glacial maximum. Figure 5 Geological Record of Oxidation state. Anoxia - Probably low O2 (except see Rosing and Frei 2004) before 2.9 Ga Oxic - Oxygen-rich air after 2.3 Ga InterOxia – Between 2.9 Ga and 2.3 Ga. After the onset of oxygenic photosynthesis, but with anoxic atmosphere (Kharecha et al., 2005), though note strong dissenting ‘oxic’ view (Ohmoto, 1997). 27