Synthesis of 2-bromo-2-methyl-propionic acid 2,5-dioxo
Synthesis of 2-bromo-2-methyl-propionic acid 2,5-dioxo-pyrrolidin-1-yl ester (1)
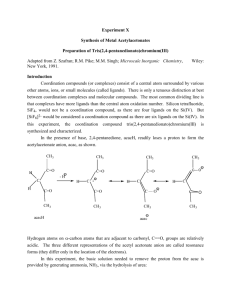
SCHEME 1 Structure of the NHS - initiator 2-bromo-2-methyl-propionic acid 2,5-dioxo-pyrrolidin-1-yl ester (1)
According to reference to a solution of 15.00 g (89.8 mmol) 2-bromo-2-methyl-propionic acid (15.00 g, 89.8 mmol) in 40 mL CH
2
Cl
2
12.4 g (107.7 mmol) N-hydroxysuccin-imide was added under stirring at 0°C. Then 23.2 g (112.4 mmol) dicyclohexylcarbodiimide was added. The reaction mixture was allowed to heat up to 25°C and was stirred for 20 hours at this temperature. After 20 hours the solution was filtered and the residue was washed with CH
2
Cl
2
. The product was received by precipitation in 200 mL ice-cold n-hexane. The product was filtered and washed with ice-cold nhexane to receive 2-bromo-2-methyl-propionic acid 2,5-dioxo-pyrrolidin-1-yl ester as white crystals
(18.25 g, 77.0 %). IR (ATR;
[cm -1 ]): 2944, 1810, 1777, 1726, 1538, 1459, 1424, 1369, 1251, 1202,
1073, 1010, 991, 923, 856, 809, 733, 641, 599, 552, 473.
4H, H-5), 2.10 (s, 6H, H-1). 13
1 H-NMR (500 MHz, CDCl
3
,
/ppm): 2.86 (s,
C-NMR (125 MHz, CDCl
3
,
/ppm): 168.6 (C-4), 167.6 (C-3), 55.9 (C-2),
30.7 (C-1), 26.0 (C-5). Anal. calcd for C
8
H
10
N
1
O
4
Br
1
: C 36.38, H 3.82, N 5.30; found: C 36.83, H 3.83, N
5.76.
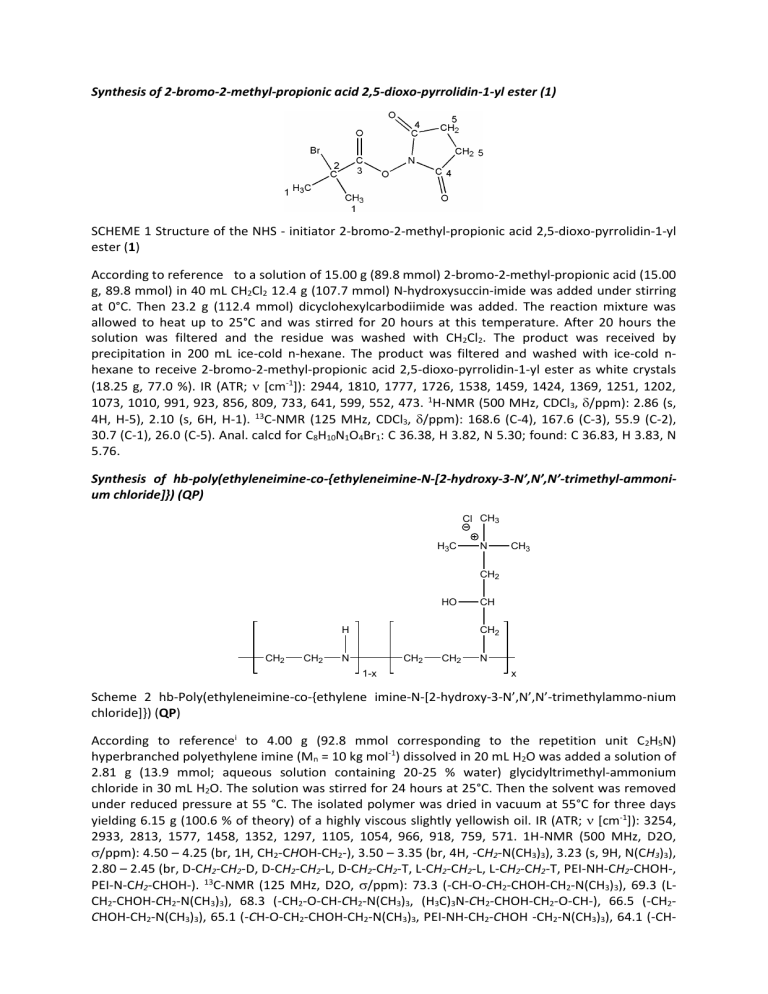
Synthesis of hb-poly(ethyleneimine-co-{ethyleneimine-N-[2-hydroxy-3-N’,N’,N’-trimethyl-ammoni-
um chloride]}) (QP)
Cl CH
3
H
3
C N CH
3
HO
CH
2
CH
H CH
2
CH
2
CH
2
N CH
2
CH
2
N
1-x x
Scheme 2 hb-Poly(ethyleneimine-co-{ethylene imine-N-[2-hydroxy-3-N’,N’,N’-trimethylammo-nium chloride]}) (QP)
According to reference i to 4.00 g (92.8 mmol corresponding to the repetition unit C
2
H
5
N) hyperbranched polyethylene imine (M n
= 10 kg mol -1 ) dissolved in 20 mL H
2
O was added a solution of
2.81 g (13.9 mmol; aqueous solution containing 20-25 % water) glycidyltrimethyl-ammonium chloride in 30 mL H
2
O. The solution was stirred for 24 hours at 25°C. Then the solvent was removed under reduced pressure at 55 °C. The isolated polymer was dried in vacuum at 55°C for three days yielding 6.15 g (100.6 % of theory) of a highly viscous slightly yellowish oil. IR (ATR;
[cm -1 ]): 3254,
2933, 2813, 1577, 1458, 1352, 1297, 1105, 1054, 966, 918, 759, 571. 1H-NMR (500 MHz, D2O,
/ppm): 4.50 – 4.25 (br, 1H, CH
2
-CHOH-CH
2
-), 3.50 – 3.35 (br, 4H, -CH
2
-N(CH
3
)
3
), 3.23 (s, 9H, N(CH
3
)
3
),
2.80 – 2.45 (br, D-CH
2
-CH
2
-D, D-CH
2
-CH
2
-L, D-CH
2
-CH
2
-T, L-CH
2
-CH
2
-L, L-CH
2
-CH
2
-T, PEI-NH-CH
2
-CHOH-,
PEI-N-CH
2
-CHOH-). 13 C-NMR (125 MHz, D2O,
/ppm): 73.3 (-CH-O-CH
2
-CHOH-CH
2
-N(CH
3
)
3
), 69.3 (L-
CH
2
-CHOH-CH
2
-N(CH
3
)
3
), 68.3 (-CH
2
-O-CH-CH
2
-N(CH
3
)
3
, (H
3
C)
3
N-CH
2
-CHOH-CH
2
-O-CH-), 66.5 (-CH
2
-
CHOH-CH
2
-N(CH
3
)
3
), 65.1 (-CH-O-CH
2
-CHOH-CH
2
-N(CH
3
)
3
, PEI-NH-CH
2
-CHOH -CH
2
-N(CH
3
)
3
), 64.1 (-CH-
O-CH
2
-CHOH-CH
2
-N(CH
3
)
3
), 63.7 (PEI-CH
2
-CHOH-CH
2
-N(CH
3
)
3
), 55.9 (D-CH
2
-CH
2
-NH
2
), 54.3 (-N(CH
3
)
3
),
53.2 (D-CH
2
-CH
2
-L), 51.2 (D-CH
2
-CH
2
-D), 50.6 (L-CH
2
-CH
2
-NH
2
), 47.8 (L-CH
2
-CH
2
-L), 45.7 (L-CH
2
-CH
2
-D),
39.9 (NH
2
-CH
2
-CH
2
-L), 37.9 (NH
2
-CH
2
-CH
2
-D). Anal. calcd for [C
2
H
4
N]
1-x
[C
8
H
19
ClN
2
O] x
, x = 0.15: C 52.92,
H 10.87, N 24.47; found: C 43.72, H 10.91, N 20.06. i
. R. Komban, R. Beckmann, S. Rode, S. Ichilmann, A. Kühnle, U. Beginn, M. Haase,
Langmuir
2011, 27, 10174 – 10183.