GCE “A” Level H2 Chemistry Nov 2008 Paper 1
advertisement
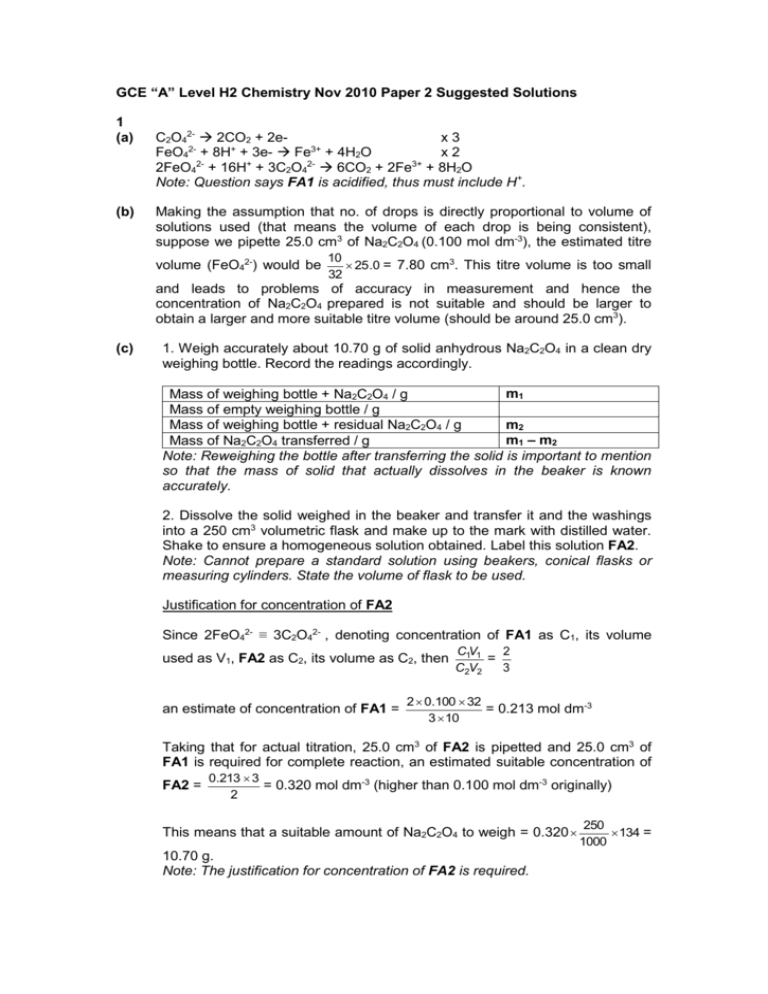
GCE “A” Level H2 Chemistry Nov 2010 Paper 2 Suggested Solutions 1 (a) (b) C2O42- 2CO2 + 2ex3 FeO42- + 8H+ + 3e- Fe3+ + 4H2O x2 2FeO42- + 16H+ + 3C2O42- 6CO2 + 2Fe3+ + 8H2O Note: Question says FA1 is acidified, thus must include H+. Making the assumption that no. of drops is directly proportional to volume of solutions used (that means the volume of each drop is being consistent), suppose we pipette 25.0 cm3 of Na2C2O4 (0.100 mol dm-3), the estimated titre volume (FeO42-) would be 10 25 .0 = 7.80 cm3. This titre volume is too small 32 and leads to problems of accuracy in measurement and hence the concentration of Na2C2O4 prepared is not suitable and should be larger to obtain a larger and more suitable titre volume (should be around 25.0 cm3). (c) 1. Weigh accurately about 10.70 g of solid anhydrous Na2C2O4 in a clean dry weighing bottle. Record the readings accordingly. m1 Mass of weighing bottle + Na2C2O4 / g Mass of empty weighing bottle / g m2 Mass of weighing bottle + residual Na2C2O4 / g m1 – m2 Mass of Na2C2O4 transferred / g Note: Reweighing the bottle after transferring the solid is important to mention so that the mass of solid that actually dissolves in the beaker is known accurately. 2. Dissolve the solid weighed in the beaker and transfer it and the washings into a 250 cm3 volumetric flask and make up to the mark with distilled water. Shake to ensure a homogeneous solution obtained. Label this solution FA2. Note: Cannot prepare a standard solution using beakers, conical flasks or measuring cylinders. State the volume of flask to be used. Justification for concentration of FA2 Since 2FeO42- ≡ 3C2O42- , denoting concentration of FA1 as C1, its volume used as V1, FA2 as C2, its volume as C2, then an estimate of concentration of FA1 = 2 C1V1 = 3 C2V2 2 0.100 32 = 0.213 mol dm-3 3 10 Taking that for actual titration, 25.0 cm3 of FA2 is pipetted and 25.0 cm3 of FA1 is required for complete reaction, an estimated suitable concentration of FA2 = 0.213 3 = 0.320 mol dm-3 (higher than 0.100 mol dm-3 originally) 2 This means that a suitable amount of Na2C2O4 to weigh = 0.320 10.70 g. Note: The justification for concentration of FA2 is required. 250 134 = 1000 3. Pipette 25.0 cm3 of FA2 into a conical flask and add about an equal volume of 1 mol dm-3 sulfuric acid (an excess), using a measuring cylinder. Note: Must mention acifification of solution and in an excess, and cannot use HCl. Also, the pippetted solution should be placed in conical flask, not beakers. 4. Titrate the above solution with FA1, placed in a burette. No external indicator is required since the the colour change at the end-point is from colourless (almost) (due to the excess FA2 and Fe3+) to pale pink (due to an excess drop of FA1). 5. Repeat titration as many times as necessary till consistent results (within 0.10 cm3 of each other) obtained. Record the results accordingly. Titration no. Rough Accurate 1 2 Final burette reading / cm3 Initial burette reading / cm3 Volume of titre used / cm3 Tick for consistent readings Summary: 25.0 cm3 of acidified FA2 required __________ cm3 of FA1 for complete reaction. (d) Amount of FA2 (C2O42-) = y M mol 1000 Since 2FeO42- ≡ 3C2O42-, amount of FA1 in x cm3 = Concentration of FA1 = (e) 2 (a) (b) 2 y M 3 1000 1000 2 y 2 yM M = mol dm-3 x 3 1000 3x Na2C2O4 and FeO4- is extremely toxic and should not be left in contact with the skin. Avoid spilling when weighing. I. H2SO4 + NaBr HBr + NaHSO4 II. CH3CH2CH2CH2OH + HBr CH3CH2CH2CH2Br + H2O Note: CH3CH2CH2CH2OH ≡ NaBr 35 = 0.340 mol 103 25 0.81 Amount of CH3CH2CH2CH2OH = = 0.274 mol < 0.340 mol 74 Amount of NaBr = Therefore NaBr is in excess. (c) (i) (ii) Protonation of H2O by conc H2SO4 (strong Bronsted acid) when H2SO4 is being diluted. Note: No credit if just say “an exothermic reaction occurs”. Inorganic by-product: Br2 Equation: H2SO4 + 2HBr Br2 + SO2 + 2H2O (accept equivalent reaction of H2SO4 with NaBr) Organic by-product: CH3CH2CH=CH2 (dehydration of butan-1-ol) Note: No credit if just write “butene” or C4H8. Equation: CH3CH2CH2CH2OH CH3CH2CH=CH2 + H2O (if equation involves H2SO4 on LHS, RHS must also have H2SO4 or its equivalent form for balancing.) (d) (e) (f) (g) (i) Almost all of organic reactions requires breaking of covalent bonds, and these in general require high amounts of energy to overcome. Water and butan-1-ol. They have closest b.p. to 1-bromobutane (118 °C), and will be distilled off together. Lower layer. 1-bromobutane has a higher density than aqueous solution (largely water). Note: No credit for comparing Mr. butan-1-ol (clue given in question: HCl is a strong acid, as a Bronsted acid, it would lose H+ to protonate butan-1-ol.) (ii) CH3CH2CH2CH2OH + HCl CH3CH2CH2CH2OH2+ + ClNote: The product is not 1-chlorobutane because this will not help to explain why it is more soluble in water than the original reactant. (iii) Product is ionic, and hence can form ion-dipole interactions between product and water, and this process is much more exothermic than formation of hydrogen bonding (limited due to non-polar alkyl chain) between butan-1-ol and water. Note: No credit for answering in terms of van der waals’ forces formed. (h) impurity: concentrated HCl (clue given in question: at step 7, “release pressure at intervals” – suggest gas is produced as by-product) equation: 2HCl + Na2CO3 2NaCl + CO2 + H2O (i) water (clue: anhydrous – lack of water) (j) from 100 to 110 ºC (note: pure 1-bromobutane boils at 102 °C) 3 (a) Photochemical smog – leads to difficulty in breathing etc Note: No credit for answering formation of acid rain – already mentioned! (b) Plot graph of initial rate against (PNO)2 or initial rate against PNO. Graphs must pass through origin. (c) 2nd order. For graph of initial rate against (PNO)2 obtained in (b) is a straight line with positive gradient, suggesting that rate is directly proportional to (PNO)2 OR For graph of initial rate against PNO obtained in (b), as PNO is doubled, rate is increased fourfold (show evidence in graph plotted.) (No credit if say since the graph is not a straight line the reaction must be 2nd order wrt PNO.) Note: Plot graph of initial rate against (PNO)2 is more straightforward. (d) 1st order. Gradient of graph as in (b) is equivalent to k(PO2)m, where m denotes order of reaction with respect to O2. Since on halving partial pressure of O2, gradient is halved, this implies that rate should be directly proportional to partial pressure of O2. (e) (i) rate = k(PNO)2PO2 (Note: No credit by writing in terms of concentrations.) (ii) Units of k = N-2 m4 s-1 (Note: Units of rate is N m-2 s-1) 4 (a) (i) magnitude of lattice energy decreases (note: actually insignificant decrease) (ii) L.E. qq . Since both charges (M2+, SO42-) and ionic radius of sulfate are r r consistent, the determining factor is the ionic radius of M2+, and from Data Booklet, the ionic radius increases from Mg2+ to Ba2+ (0.065 to 0.135 nm). This means the ionic bond strength between the oppositely charged ions gets weaker due to less close approach. (b) (i) δ+ δ+ δ+ δ+ δδ- Dotted lines denote ion-dipole interactions (ii) ΔHhyd q . Obviously the charge is constant (+2), and from Data Booklet, r the ionic radius increases from Mg2+ to Ba2+. The charge on cation is less concentrated as ionic radius increases (decreasing charge density), and hence there is decrease in ability to attract H2O molecules (decrease in polarising power) strongly (the ion-dipole attraction energy decreases). Note: No credit if argue in terms of ΔG and ΔH. (c) (i) nucleophilic addition (No credit for just stating “addition”.) (ii) hydrolysis (look at the products formed, overall there is addition of H-OH.) (d) (e) 5 (a) (i) The disruption of secondary and/or tertiary structure of protein molecules due to disruption and/or modification of various R (residual) group interactions by adding weak acids, heating, enzymes etc. however, the exact amino acid sequence that makes up the protein (primary structure) remains intact. (ii) Heating to a high temperature is a possiblity (question says gelatin is prepared in the food and pharmaceutical industries). The weaker R group interactions such as hydrogen bonding or p.d.-p.d. will get disrupted. No credit for stating hydrolysis of collagen – not denaturation! (b) 6 (c) (i) (ii) Question asks for amino acid molecules, not repeat units. (d) (i) Extended heating for a few hours with 6 mol dm-3 HCl or 6 mol dm-3 NaOH. (ii) (iii) Monomer of proteins: amino acids – each contains both –CO2H and –NH2 group. Monomer of Kevlar: dicarboxylic acids and diamines separately Question is not asking for difference between monomers that make up Kevlar (e) HOCH2CO2CH(CH3)CO2H and CH3CH(OH)CO2CH2CO2H (f) (i) (ii) hydrolysis of ester to reform carboxylic acid and alcohol formation of ion-dipole interactions between carboxylate ions and water, and hydrogen bonding between –OH groups and water.







