A short review on Basic Chemistry according to Dr

Read this article do one or both of the following activities for extra credit.
1) Note 5 facts that have not been discussed in class and present them to the class.
2) Look more deeply into one of the facts that interests you and write a paragraph or more explaining what you have discovered.
A short review on Basic Chemistry according to Dr. Gloria G.
Salandanan, a college Science professor.
1.
The smallest particle of matter that exhibits the properties of that matter is called molecules.
2.
Molecules are made up of atoms.
3.
According to atomic theory, all substances consist of small particles called atoms.
4.
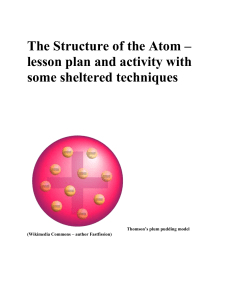
The atom has a central mass called nucleus in which are located the protons and the nucleus. The nucleus is surrounded by orbits on which the electrons travel.
5.
A proton carries a positive electric charge and has a mass identical to a hydrogen atom.
6.
An electron carries a negative electric charge and has a mass about 1/2000th that of a proton or neutron.
7.
A neutron carries no electric charge and has a mass nearly the same as that of a proton.
8.
The atom is the smallest unit in an element. A total of 107 elements have been identified.
9.
The atomic number refers to the number of positive charges in a nucleus.
10.
The atom is electrically neutral. The number of positive charges in it is the same as the negative charges. An atom contains the same number of protons and neutrons.
11.
The mass number of an atom is equal to the number of protons plus the number of neutrons.
12.
Each orbit represents an amount of energy since energy is required to hold the electrons in orbit. The orbit close to nucleus has less energy than the farther orbits.
13.
An atom carrying an electrical charge is called an ion. If an electron is removed, the atom becomes positively charged.
14.
Atoms of the same element with the same atomic number but with different mass numbers are called isotopes.
15.
A nucleus is stable if there is one neutron for every proton or, sometimes, there are slightly more neutrons than protons.
16.
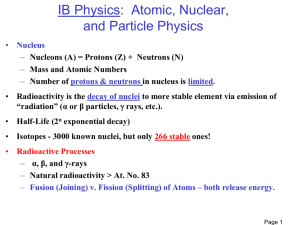
When a substance emits radiation in he form of energy or particles, it is said to be radioactive.
17.
Natural radioactivity is the spontaneous emission of radiation occurring in nature, artificial radioactivity results when the nucleus of an atom is bombarded.
18.
Three types of radiations are alpha, beta, and gamma radiations. An alpha particle is a positively charged particle with a mass and charge of two protons and two neutrons. A beta particle is a charged particle with a mass and charge of 1 electron. Gamma rays are similar to X-rays. They have neither mass nor charge.
19.
Alpha particles can be stopped by a sheet of paper. They can penetrate just the surface of the skin. Beta particles can penetrate one or two centimeters of human tissue. They cause damage to the cells. Gamma rays are highly
penetrating and can pass through the human body.
20.
The natural sources of radiation are the sun, materials on the earth’s surface, and elements taken through food, water, and air. The artificial sources of radiation are medical diagnosis treatment, living quarters, television sets, and fallout from nucleus tests.
21.
Radioisotopes are isotopes that emit radiation. Today they are widely used in improving agriculture techniques. In medicine, they are used in diagnosing and treating diseases.
Radioisotopes are also used in improving industrial process.