Attorney Docket No. 31951-773.601 CLAIMS WHAT IS CLAIMED IS
advertisement
Attorney Docket No. 31951-773.601
CLAIMS
WHAT IS CLAIMED IS:
1.
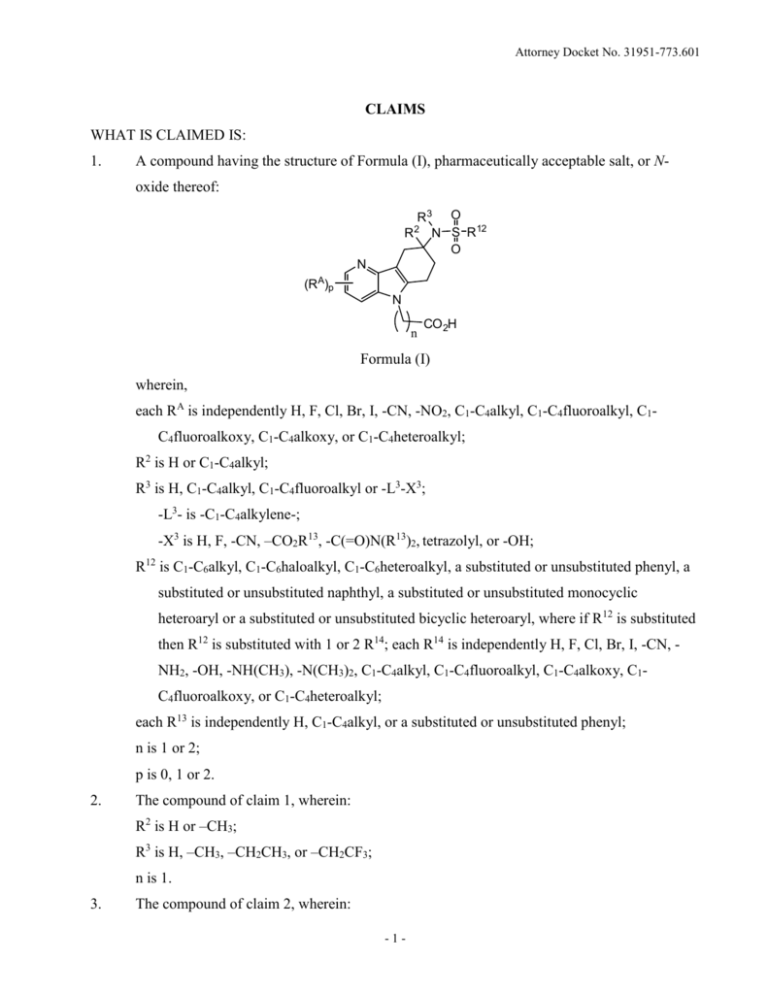
A compound having the structure of Formula (I), pharmaceutically acceptable salt, or Noxide thereof:
R3 O
R N S R12
O
2
N
(RA)p
N
n
CO 2H
Formula (I)
wherein,
each RA is independently H, F, Cl, Br, I, -CN, -NO2, C1-C4alkyl, C1-C4fluoroalkyl, C1C4fluoroalkoxy, C1-C4alkoxy, or C1-C4heteroalkyl;
R2 is H or C1-C4alkyl;
R3 is H, C1-C4alkyl, C1-C4fluoroalkyl or -L3-X3;
-L3- is -C1-C4alkylene-;
-X3 is H, F, -CN, –CO2R13, -C(=O)N(R13)2, tetrazolyl, or -OH;
R12 is C1-C6alkyl, C1-C6haloalkyl, C1-C6heteroalkyl, a substituted or unsubstituted phenyl, a
substituted or unsubstituted naphthyl, a substituted or unsubstituted monocyclic
heteroaryl or a substituted or unsubstituted bicyclic heteroaryl, where if R12 is substituted
then R12 is substituted with 1 or 2 R14; each R14 is independently H, F, Cl, Br, I, -CN, NH2, -OH, -NH(CH3), -N(CH3)2, C1-C4alkyl, C1-C4fluoroalkyl, C1-C4alkoxy, C1C4fluoroalkoxy, or C1-C4heteroalkyl;
each R13 is independently H, C1-C4alkyl, or a substituted or unsubstituted phenyl;
n is 1 or 2;
p is 0, 1 or 2.
2.
The compound of claim 1, wherein:
R2 is H or –CH3;
R3 is H, –CH3, –CH2CH3, or –CH2CF3;
n is 1.
3.
The compound of claim 2, wherein:
-1-
Attorney Docket No. 31951-773.601
R2 is H;
R12 is a substituted or unsubstituted phenyl, or a substituted or unsubstituted 6-membered
monocyclic heteroaryl, where if R12 is substituted then R12 is substituted with 1 or 2 R14;
each R14 is independently H, F, Cl, Br, I, -CN, -NH2, -OH, -NH(CH3), -N(CH3)2, -CH3, CF3, -OCH3, or -OCF3.
4.
The compound of claim 3, wherein the compound of Formula (I) has the structure of Formula
(II):
(R14) r
R3 O
N S
O
N
A
(R )p
N
CO2H
Formula (II)
wherein:
r is 0, 1 or 2.
5.
The compound of claim 4, wherein:
each RA is independently H, F, Cl, Br, -CN, -CH3, -CF3, -OCF3, or -OCH3;
each R14 is independently H, F, Cl, Br, -CN, -CH3, -CF3, -OCF3, or -OCH3;
r is 1.
6.
The compound of claim 5, wherein the compound of Formula (II) has the structure of
Formula (III):
R3 O
N S
O
R14
N
A
(R )p
N
CO 2H
7.
Formula (III).
The compound of claim 6, wherein:
R3 is –CH3;
R14 is F;
p is 0.
8.
The compound of claim 5, wherein the compound of Formula (II) has the structure of
Formula (IV):
-2-
Attorney Docket No. 31951-773.601
R3 O
N S
O
R14
N
A
(R )p
N
CO 2H
9.
Formula (IV).
The compound of claim 8, wherein:
R3 is –CH3;
R14 is F;
p is 0.
10.
The compound of claim 1 selected from:
{8-[(4-fluoro-benzenesulfonyl)-amino]-6,7,8,9-tetrahydro-pyrido[3,2-b]indol-5-yl}-acetic
acid;
{8-[(4-fluoro-benzenesulfonyl)-methyl-amino]-6,7,8,9-tetrahydro-pyrido[3,2-b]indol-5-yl}acetic acid;
(R)-{8-[(4-fluoro-benzenesulfonyl)-methyl-amino]-6,7,8,9-tetrahydro-pyrido[3,2-b]indol-5yl}-acetic acid;
(S)-{8-[(4-fluoro-benzenesulfonyl)-methyl-amino]-6,7,8,9-tetrahydro-pyrido[3,2-b]indol-5yl}-acetic acid;
{8-[(4-fluoro-benzenesulfonyl)-methyl-amino]-6,7,8,9-tetrahydro-pyrido[3,2-b]indol-5-yl}propionic acid;
{8-methyl-8-[(4-fluoro-benzenesulfonyl)-methyl-amino]-6,7,8,9-tetrahydro-pyrido[3,2b]indol-5-yl}-acetic acid;
{8-[(4-chloro-benzenesulfonyl)-methyl-amino]-6,7,8,9-tetrahydro-pyrido[3,2-b]indol-5-yl}acetic acid;
{8-[(4-methoxy-benzenesulfonyl)-methyl-amino]-6,7,8,9-tetrahydro-pyrido[3,2-b]indol-5yl}-acetic acid;
{8-[(4-cyano-benzenesulfonyl)-methyl-amino]-6,7,8,9-tetrahydro-pyrido[3,2-b]indol-5-yl}acetic acid;
{8-[(4-trifluoromethyl-benzenesulfonyl)-methyl-amino]-6,7,8,9-tetrahydro-pyrido[3,2b]indol-5-yl}-acetic acid;
{8-[(3-fluoro-benzenesulfonyl)-methyl-amino]-6,7,8,9-tetrahydro-pyrido[3,2-b]indol-5-yl}acetic acid;
-3-
Attorney Docket No. 31951-773.601
{8-[(2-fluoro-benzenesulfonyl)-methyl-amino]-6,7,8,9-tetrahydro-pyrido[3,2-b]indol-5-yl}acetic acid;
{8-[(3,4-difluoro-benzenesulfonyl)-methyl-amino]-6,7,8,9-tetrahydro-pyrido[3,2-b]indol-5yl}-acetic acid;
{8-[(6-methoxy-pyridin-3-ylsulfonyl)-methyl-amino]-6,7,8,9-tetrahydro-pyrido[3,2-b]indol5-yl}-acetic acid;
{8-[(6-fluoro-pyridin-3-ylsulfonyl)-methyl-amino]-6,7,8,9-tetrahydro-pyrido[3,2-b]indol-5yl}-acetic acid; and
{8-[(4-fluoro-benzenesulfonyl)-(1,1,1-trifluoroeth-2-yl)-amino]-6,7,8,9-tetrahydropyrido[3,2-b]indol-5-yl}-acetic acid.
11.
A pharmaceutical composition comprising a therapeutically effective amount of a compound
of any one of claims 1-10, or pharmaceutically acceptable salt, N-oxide, or prodrug thereof,
and at least one pharmaceutically acceptable inactive ingredient selected from
pharmaceutically acceptable diluents, pharmaceutically acceptable excipients, and
pharmaceutically acceptable carriers.
12.
The pharmaceutical composition of claim 11, wherein:
(a) the pharmaceutical composition is formulated for intravenous injection, oral
administration, inhalation, nasal administration, topical administration, ophthalmic
administration or otic administration; or
(b) the pharmaceutical composition is a tablet, a pill, a capsule, a liquid, an inhalant, a nasal
spray solution, a suppository, a suspension, a gel, a colloid, a dispersion, a suspension, a
solution, an emulsion, an ointment, a lotion, an eye drop or an ear drop.
13.
The compound of any of claims 1-10 for use in treating a respiratory disease or condition, an
allergic disease or condition, or an inflammatory disease or condition in a mammal.
14.
The compound of any of claims 1-10 for use in treating asthma, chronic obstructive
pulmonary disease (COPD), or allergic rhinitis in a mammal.
-4-
Attorney Docket No. 31951-773.601
CYCLOALKANE[B]AZAINDOLE ANTAGONISTS OF PROSTAGLANDIN D2
RECEPTORS
ABSTRACT
Described herein are compounds that are antagonists of PGD2 receptors. Also described are
pharmaceutical compositions that include the compounds described herein, and methods of using
such antagonists of PGD2 receptors, alone or in combination with other compounds, for treating
respiratory, cardiovascular, and other PGD2-dependent or PGD2-mediated conditions or diseases.
-5-








