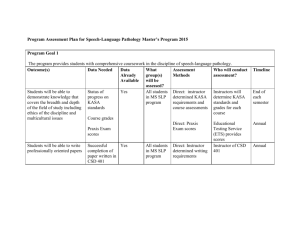
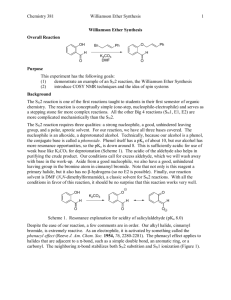
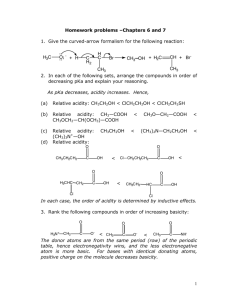
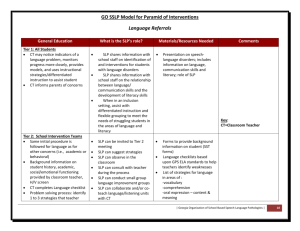
032410 substitution and elimination reactions
advertisement

Substitution & Elimination Supplemental Instruction Iowa State University Leader: Course: Instructor: Date: Chelsea Chem 231 Jeffries-El 3/24/10 Consider the reaction: CH3CH2Br + CH3OH CH3CH2-OCH3 What type of reaction is this? (SN1, SN2, E1, or E2) SN2 What is the substrate? CH3CH2Br What is the nucleophile? CH3OH What is added to the substrate (might be different than the nucleophile)? CH3OWhat is the leaving group? BrWhat happens to the reaction rate if… 1. The substrate is changed to CH3Br? increases 2. The substrate is changed to (CH3)2CH2Br? decreases 3. The leaving group is changed to Cl-? decreases 4. The leaving group is changed to I-? increases 5. The nucleophile is changed to NaOCH3? increases 6. The concentration of CH3OH is doubled? increases 7. The concentration of CH3CH2Br is halved? decreases 8. Both 6 and 7 happen at the same time? Stays the same Which of these 8 will change the overall product of the reaction? (only 1 & 2) What are the products of these substitution reactions? Are they SN1 or SN2? CH3CH2OH + PBr3 CH3CH2Br (SN2) CH3CH2CH2CH2Br + NaCN CH3CH2CH2CH2CN + NaBr (SN2) CH3Br + NaOC(CH3)3 CH3OC(CH3)3 + NaBr (SN2) CH3CH2CH2I + NaN3 CH3CH2CH2N3 + NaI (SN2) CH3CH2I + NaSH CH3CH2SH + NaI (SN2) CH3CH2CHCH3 + CH3OH subst. product (inverted stereochem) & 2 elim. Products Br (SN2 & E2) CH3Br + NaOCOCH3 CH3OCOCH3 + NaBr (SN2) What are the products of these elimination reactions? (CH3)3CBr + NaOCH3 CH2=C(CH3)2 (E2) CH3CH2CHCH3 + CH3OH subst. product (inverted stereochem) & 2 elim. Products Br (SN2 & E2)