Isotopes Worksheet
advertisement

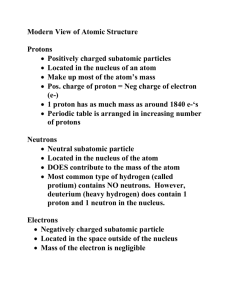
ISOTOPES – PROBLEM SET IPC – Mr. Coburn Introduction Atoms of a given element which have the same number of protons but different numbers of neutrons are called isotopes. Thus, isotopes have the same position in the periodic table, the same chemical properties and the same atomic charge. The simplest example of an atom with different isotopes is hydrogen. The three isotopes of hydrogen are shown below: The increasing number of neutrons in the nucleus of the hydrogen atom adds mass to the atom and thus each isotope of a given element has a different mass. For the isotopes of hydrogen, 1H (or hydrogen-1), 2H (or hydrogen-2) and 3H (or hydrogen-3) represent Protium (usually just referred to as hydrogen), Deuterium and Tritium, respectively. Most of the light elements contain different proportions of at least two isotopes. Usually one isotope is the predominantly abundant isotope. For example, the average abundance of 12C is 98.89%, while the average abundance for 13C is 1.11%. Calculating Atomic Mass The calculation of the atomic mass of an element is performed using the relative abundance data from the isotope of each atom. Atomic Mass = [(% abundance of isotope)(mass of isotope)] + [(% abundance of isotope)(mass of isotope)] + [….] 100 For example: The natural abundance for boron isotopes is 19.9% 10B (10.013 amu) and 80.1% 11B (11.009 amu). Calculate the atomic mass of boron. Average atomic mass = [(19.9%)(10.013)] + [(80.1%)(11.009)] 100 = 10.811 amu Part I – Answer the following questions 1. What is an isotope? _____________________________________________________________ ________________________________________________________________________________ 2. What does the number next to isotopes signify? _______________________________________ ________________________________________________________________________________ 3. How can you tell isotopes apart? ___________________________________________________ ________________________________________________________________________________ Part II - Use the periodic table and the information provided to complete any missing information (including isotope numbers) for the charts below. Chromium-52 Chromium-54 # of protons # of protons # of neutrons # of neutrons # of electrons # of electrons Iron- Iron- # of protons # of protons # of neutrons # of neutrons 29 31 # of electrons # of electrons # of protons # of protons Selenium-35 Selenium-36 Iodine- Iodine- 73 75 146 147 79 # of neutrons # of neutrons 117 # of electrons 118 # of electrons 92 Part III - Calculate the atomic mass for each element based on the information provided. 1. Calculate the average atomic mass of iron if its abundance in nature is 15% iron-55 and 85% iron-56. 2. What is the average atomic mass of silicon if 92.21 % of its atoms have a mass of 27.977 amu, 4.07 % have a mass of 28.976 amu, and 3.09 % have a mass of 29.974 amu? 3. Calculate the average atomic mass for neon if its abundance in nature is 90.5% neon-20 (19.922 amu), 0.3% neon-21 (20.994 amu), and 9.2% neon-22 (21.991 amu). 4. Calculate the average atomic mass of silver if 13 out of 25 atoms are silver-107 and 12 out of 25 atoms are silver-109. 5. Calculate the average atomic mass of chromium. Isotope Mass (amu) Chromium – 50 49.946 Chromium – 52 51.941 Chromium – 53 52.941 Chromium – 54 53.939 Relative Abundance 0.043500 0.83800 0.095000 0.023500 6. Boron has three naturally occurring isotopes: boron-10, boron-11, and boron-12. If the average atomic mass of boron is 10.811 amu, which isotope is the most abundant? How do you know? 7. Bromine has two isotopes. Bromine-79 occurs at a relative abundance of 50.69% and Bromine-81 has a relative abundance of 49.31%. What is the atomic mass of bromine? 8. Determine the atomic mass of Chlorine with the following data: i. Cl-35 with a relative abundance of 75.77% ii. Cl-37 with a relative abundance of 24.23%