Topic: HCV RNA Viral Load Testing Date: 7/22/05
advertisement

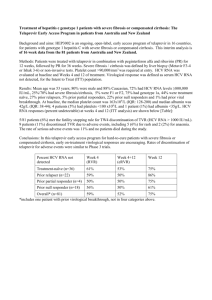
Technical Laboratory Bulletin Topic: HCV RNA Viral Load Testing Test Name: Test Code: Method: Changes: Date: 7/22/05 HCV RNA Viral Load HCVVL Bayer dDNA Testing will now be performed at NorDx using the FDA approved bDNA method. Previously the testing was sent to Labcorp which uses the Roche COBAS Taqman ASR. 3 mL whole blood in an EDTA tube. See below for specimen Specimen: handling. Test Schedule: Tuesday. Analytical time 2 days. Change: Effective July 29, 2005, NorDx will begin testing HCV RNA Viral Load specimens in-house using the Bayer branched DNA (bDNA) methodology. The dynamic range of the assay is 615 to 7,690,000 IU/mL. Indications: The HCV RNA Viral Load assay is a signal amplification nucleic acid probe assay for the direct quantitation of Hepatitis C Virus RNA in plasma of HCV infected individuals using the VERSANT bDNA System 340. The assay is not intended for screening, confirmatory testing, establishment of HCV infection or detection or measurement of antibody to HCV. Specimen Processing: NOTE: RNA is especially vulnerable to degradation if not processed according to protocol. A. COLLECTION 1. Peripheral blood collected in 4mL or 7 mL K3EDTA(0.15%v/v solution) tube (eg. Vacutainer Becton Dickenson Vacutainer Systems, Franklin Lakes, NJ) a. Minimum volume: 3 mL (Does not allow for repeat testing) b. Dedicated tube only- no sharing of specimen for other tests (assay is extremely vulnerable to RNA or enzymatic contamination). c. Maintain at room temperature (18-30oC). d. Process as below within 4 hours of collection. e. Assay is not considered diagnostic for HCV infection. Patient must have documented positive antibody response or qualitative HCV RNA or HCV DNA test result. B. PROCESSING WHOLE BLOOD 1. Centrifuge specimen at 1000 x g for 10-15 minutes within 4 hours of blood collection. Do not clarify specimen further. 2. Aseptically remove and aliquot plasma (do not disturb white cell layer) into sterile unbreakable tubes (duplicate aliquots of at least 0.75 mL plasma are advised to avoid freeze-thaw of specimen in case of repeat testing). 3. Immediately freeze plasma at -60oC to -80oC within 30 minutes of separation. 4. Specimens may be stored at –20oC in a non-frost-free freezer for up to 72 hours. 5. Avoid repeated freeze/thaw of the samples. 6. Moderately hemolyzed or lipemic are acceptable. Result Reporting: HCV RNA Viral load is reported in IU/mL. Dynamic range of the assay is from 615 to 7,690,000 IU/mL. References: Sheridan P, Detmer J, Hunt W, Chan C, Wright T, Wilber J, Neuwald P, Urdea M, Sanchez-Pescador R. Detection of hepatitis C virus RNA in human sera by a quantitative branched DNA amplification assay. American Society for Microbiology, New Orleans, LA, May 16-20, 1992. Abstract S53. Additional Information: Contact Gene R. Putz, Ph.D., Laboratory Director, NorDx, (207) 885-7809 or Email at putzg@mmc.org