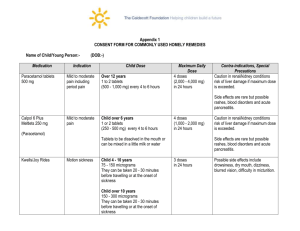
GENERIC_DESC
advertisement

PRODUCT DESCRIPTION TRADE NAME DOSAGE FORM/STRENGTH Acetazolamide Diamox Injection 500mg Syrup 25mg/mL Tablet 250mg Acetyl Salicylic Acid Aspirin Aspirin Pedia Juspirin Tablet 81mg Tablet 300mg Acetylcholine Chloride Miochol Injection 1% Acetylcysteine Parvolex Inj. 2g/10mL Actinomycin Dactinomycin Cusiviral Acyclovir Cyclovax Zovirax PRODUCT INFORMATION THERAPEUTIC USE: Anticonvulsant, Antiglaucoma, Carbonic Anhydrase Inhibitor, Cardiovascular Agent, Musculoskeletal Agent, RenalUrologic Agent Sulfonamide, Urinary Stone Agent DOSE: Adult - 250 to 1000 mg ORALLY divided 1 to 4 times daily, extended-release: 500 mg ORALLY twice daily & max. dose of 1000 mg/day is recommended. Injection - Acute Angle-Closure Glaucoma, Intravenous therapy may be used for rapid relief of ocular tension in acute cases. The recommended dose for acute congestive glaucoma is 250 mg every 4 hours. Some patients may respond to 250 mg 2 times daily on a short-term basis. Pedia - initial, 4 mg/kg ORALLY daily maintenance, 8 to 30 mg/kg ORALLY daily, divided every 6 to 8 hours. Injection - Glaucoma; Adjunct, recommended dose for glaucoma in children is 5 to 10 mg/kg/dose every 6 hours or 20 to 40 mg/kg/day. CONTRA-INDICATIONS: Adrenal gland failure, chronic noncongestive angle-closure glaucoma, cirrhosis, hyponatremia/hypokalemia, hyperchloremic acidosis, hypersensitivity to acetazolamide and severe hepatic or renal disease ADVERSE EFFECTS: Angle-closure glaucoma, Metabolic acidosis, Sulfonamide adverse reaction, Including anaphylaxis, blood dyscrasias, erythema multiforme, fulminant hepatic necrosis, Stevens-Johnson syndrome, toxic epidermal necrolysis & Tinnitus THERAPEUTIC USE: Analgesic, Antipyretic, Antirrheumatic ,Central Nervous System Agent, NSAID, Platelet Aggregation Inhibitor Salicylate, DOSE: Adult - 50 to 325 mg ORALLY once daily; maximum of up to 4 g ORALLY per day in divided doses. Pedia - 12 years and older, 325 to 650 mg ORALLY every 4 hours; MAX: 3.9 grams/24 hours CONTRA-INDICATIONS: hypersensitivity to NSAIDs, children and teenagers with chickenpox or flu symptoms (risk of Reye's syndrome), syndrome of asthma, rhinitis, & nasal polyps ADVERSE EFFECTS: Angioedema, Bleeding, Bronchospasm, Gastrointestinal ulcer, Reye's syndrome and Tinnitus THERAPEUTIC USE: Cholinergic, Direct Acting Miotic DOSE: Adult - Miosis induction - 0.5 to 2mL instilled INTRAOCULARLY into the anterior chamber before or after securing one or more sutures; Pedia - safety and effectiveness in pediatric patients have not been established CONTRA-INDICATIONS: hypersensitivity to acetylcholine chloride or any component of the product ADVERSE EFFECTS: Bradyarrhythmia, With systemic absorption, Cataract, Transient, Corneal decompensation, Corneal edema, Endophthalmitis, Hypotension, With systemic absorption, Optic atrophy THERAPEUTIC USE: Acetaminophen Antidote, Amino Acid Supplement, Diagnostic Agent, Bronchial, Mucolytic DOSE: Adult & Pedia- Acetaminophen overdose, initial, 140 mg/kg ORALLY, then 70 mg/kg every 4 h for 17 doses starting 4 hr after loading dose; initial, 150 mg/kg IV in 200 mL of 5% dextrose over 60 minutes, then 50 mg/kg IV in 500mL 5% dextrose over 4 hours, followed by 100 mg/kg IV in 1000mL of 5% dextrose over 16 hours. Nebulization into a face mask, mouth piece, or tracheostomy: 1 to 10mL of the 20% solution or 2 to 20mL of the 10% solution every 2 to 6 h; usual dose 3 to 5mL of the 20% solution or 6 to 10mL of the 10% solution 3-4 times daily. ADVERSE EFFECTS: Anaphylaxis, Bronchospasm Injection 0.5mg THERAPEUTIC USE: Antibiotic DOSE: Adult & Pedia - Ewing's sarcoma of bone - 15 mcg/kg IV every day for 5 days (in combination with other chemotherapy); Gestational trophoblastic neoplasm - 12 mcg/kg IV every day for 5 days (single agent); 500 mcg IV days 1, 2 (in combination with etoposide, methotrexate, folinic acid, vincristine, cyclophosphamide, and cisplatin); CONTRA-INDICATIONS: chickenpox or herpes zoster infection; increased risk for severe generalized disease which could result in death, Hypersensitivity to dactinomycin ADVERSE EFFECTS: Anaphylaxis, Hepatotoxicity, Liver failure, Myelosuppression, Veno-occlusive disease of the liver Cream 5% Eye Oint. 3% Injection 250mg Tablet 200mg THERAPEUTIC USE: Antiviral, Guanosine Nucleoside Analog, Viral DNA Polymerase Inhibitor DOSE: Adult - Genital herpes simplex initial episode, 400 mg ORALLY 3 times a day or 200 mg ORALLY 5 times a day, for 7 to 10 days; Herpes simplex, mucosal and cutaneous - Patient immunocompromised - 5 mg/kg IV every 8 hr for 7 days; apply ointment TOPICALLY every 3 hours (6 times per day) for 7 days in sufficient quantities to adequately cover lesions. Pedia - congenital herpes simplex - birth to 3 mos of age, 10 mg/kg IV every 8 h for 10 days; doses of 15 to 20 mg/kg IV have been used; CDC recommends 20 mg/kg IV every 8 h, for 21 days for disseminated disease or 14 days for disease of the skin and mucous membranes. CONTRA-INDICATIONS:Hypersensitivity to acyclovir or valacyclovir ADVERSE EFFECTS: Agitation, Confusion, Encephalopathic changes, Hemolytic uremic syndrome, Immunocompromised patients; some fatal cases, Lethargy, Encephalopathic changes, Renal impairment, Thrombotic thrombocytopenic purpura, Immunocompromised patients; some fatal cases and Tremor. 1 PRODUCT DESCRIPTION Adenosine TRADE NAME Adenocor Adrenaline (Epinephrine) Albendazole DOSAGE FORM/STRENGTH Injection 6mg/2mL Injection 1:1000 Inj. 1:10,000 Albenda Tablet 200mg Alzental Albumin Solution Injection 20% Alfacalcidol One Alpha Cap. 0.25mcg Capsule 1mcg Drops 2mcg/mL Alfuzosin Xatral XL Tablet 10mg Allopurinol No Uric Syrup 20mg/mL Tablet 100mg Tablet 300mg PRODUCT INFORMATION THERAPEUTIC USE: Antiarrhythmic, Nutriceutical DOSE: Adult - Advanced cardiac life support - Supraventricular tachycardia - initial 6 mg IV peripheral bolus over 1 to 2 seconds, increase to 12 mg every 1 to 2 min as needed for 2 doses; MAX 12 mg/dose. Pedia - Advanced cardiac life support - Supraventricular tachycardia initial, 0.1 mg/kg/dose IV or IO, MAX 6 mg/dose; may repeat at 0.2 mg/kg increments as needed; MAX 12 mg/dose CONTRA-INDICATIONS: hypersensitivity to adenosine, second or third degree AV block, sinus node dysfunction, such as sick sinus syndrome or symptomatic bradycardia ADVERSE EFFECTS: Bradyarrhythmia, Bronchospasm, In asthmatics, Cardiac dysrhythmia, Partial atrioventricular block THERAPEUTIC USE: Adrenergic, Alkylarylamine, Anaphylaxis Agent, Anesthetic Adjunct, Antiglaucoma, Bronchodilator, Decongestant, Sympathomimetic, Vasopressor DOSE: Adult – Anaphylaxis (auto-injector) 0.3mg IM (0.3mL of a 1:1000 solution); may be repeated if severe anaphylaxis persists, (injectable solution) 0.2 to 1mg SUBQ. Pedia – Anaphylaxis (auto-injector) 0.15 to 0.3mg IM depending on body weight, a dosage of 0.01 mg/kg is recommended CONTRA-INDICATIONS: Cardiac dilatation & coronary insufficiency, concurrent use with cyclopropane or halogenated hydrocarbon anesthetic; may sensitize the heart to the arrhythmic action of sympathomimetic drugs,concurrent use with local anesthetics for injection of certain areas (eg; fingers, toes, ears); increased risk of vasoconstriction and sloughing of tissue, hypersensitivity to sympathomimetic amines, ADVERSE EFFECTS: Cardiac dysrhythmia, Hypertensive crisis, Pulmonary edema THERAPEUTIC USE: Anthelmintic, Benzimidazole DOSE: Adult & Pedia - Ancylostomiasis and necatoriasis 400 mg ORALLY as a single dose ADVERSE EFFECTS: Acute renal failure, Agranulocytosis, Aplastic anemia, Erythema multiforme, granulocytopenic disorder, Hepatotoxicity, With elevated liver enzymes Leukopenia, Pancytopenia, Stevens-Johnson syndrome, Thrombocytopenia THERAPEUTIC USE: Volume Expander DOSE: Adult - Cardiopulmonary bypass operation 5%, adjust albumin and crystalloid pump prime to achieve a hematocrit of 20% and a plasma albumin concentration of 2.5 g/100mL; Hypovolemic shock - IV, 500mL of 5% solution as rapidly as tolerated, may repeat in 30 minutes; IV, 100 to 200mL of 25% solution, may repeat in 15-30 minutes. Pedia - Hypovolemic shock - IV, 10 to 20mL/kg of 5% solution, may repeat in 30 minutes, MAX 6 g/kg/day; IV, 2.5 to 5mL of 25% solution, may repeat in 15-30 minutes CONTRA-INDICATIONS: Hypersensitivity to albumin, patients at risk for acute circulatory overload ADVERSE EFFECTCS: Immune hypersensitivity reaction THERAPEUTIC USE: Nutritive Agent, Vitamin D (class) DOSE: Adults - once daily with food. Pedia - 1mcg daily have been administered to children 2 to 17 years of age for control of secondary hyperparathyroidism; Addition of oral alfacalcidol 10 to 20 ng/kg daily to standard therapy CONTRA-INDICATIONS: Prior hypersensitivity to alfacalcidol, vitamin D (cholecalciferol, ergocalciferol), or other vitamin D metabolites (eg, calcitriol, calcifediol, calcipotriol) Hypercalcemia (exacerbation with enhanced toxicity) Other evidence of hypervitaminosis D (exacerbation) THERAPEUTIC USE: Alpha-1 Adrenergic Blocker, Benign Prostatic Hypertrophy Agent DOSE: Adult -Benign prostatic hyperplasia extended-release: 10mg ORALLY once daily. Pedia - safety and effectiveness not established in children CONTRA-INDICATIONS: concomitant use of potent CYP3A4 inhibitors such as ketoconazole, itraconazole, and ritonavir; increases blood levels of alfuzosin, hepatic insufficiency, moderate or severe; may lead to increased alfuzosin levels in the blood, and hypersensitivity to alfuzosin or to any component of the product ADVERSE EFFECTS: Angina ,Intra-operative floppy iris syndrome, Orthostatic hypotension, Prolonged QT interval, Tachycardia THERAPEUTIC USE: Anti-gout, Urinary Stone Agent, Xanthine Oxidase Inhibitor DOSE: Adult - Calcium renal calculus, recurrent 200 to 300mg ORALLY as a single or divided dose (2-3 times daily); maximum dose: 300 mg/dose; 800 mg/day. Pedia - Hyperuricemia Tumor lysis syndrome - (under 6 yrs) 50 mg ORALLY 3 times daily; (6-10 yrs) 100 mg ORALLY 3 times daily OR 300 mg ORALLY once daily ; 200 mg/m(2)/day IV starting 24-48 hours prior to initiation of chemotherapy as a single infusion OR in equally divided infusions at 6-, 8-, or 12-hour intervals ADVERSE EFFECTCS: Agranulocytosis, Anemia, Hepatotoxicity, Myelosuppression, Stevens-Johnson syndrome 2 PRODUCT DESCRIPTION TRADE NAME DOSAGE FORM/STRENGTH Alprostadil Prostin VR Injection 500mcg Alteplase Actilyse Injection 50mg Alum Hydroxide/ Magnesium Hydroxide Moxal Rialox Suspension Amethocaine HCl (Tetracaine) Amethocaine Eye Minims 0.5% Amikacin Sulphate Miacin Injection 100mg Injection 500mg AmilorideHCl/ Hydrochlorothiazide Moduretic Tablet PRODUCT INFORMATION THERAPEUTIC USE: Endocrine-Metabolic Agent, Erectile Dysfunction Agent, Prostaglandin DOSE: Adult - Erectile dysfunction, initial dose of 2.5mcg INTRACAVERNOSAL INJECTION, average dose of 2.5-60mcg; no more than 3 doses weekly with at least 24 hours between doses; or 125-1000mcg urethral SUPPOSITORY; no more than 2 doses in 24 hours; Pedia – Cyanotic congenital heart disease initial, 0.05-0.1mcg/kg/min continuous IV, maintenance 0.01-0.4mcg/kg/min CONTRA-INDICATIONS: anatomical deformation of the penis, hypersensitivity to alprostadil, leukemia, myeloma, neonatal respiratory distress syndrome, penile implant MUSE® is contraindicated for use with pregnant sexual partners unless a condom is used ADVERSE EFFECTS: Apnea in the newborn, Congestive heart failure, Disorder of bone development, Cortical proliferation of long bones, Disseminated intravascular coagulation, Prostin, Partial atrioventricular block, Priapism, Seizure, Supraventricular tachycardia, Ventricular fibrillation THERAPEUTIC USE: Thrombolytic, Tissue Plasminogen Activator DOSE: Adult - Acute myocardial infarction, Accelerated infusion: patients over 67 kg- total dose 100 mg IV, give 15 mg IV bolus, 50 mg over 30 minutes, then 35 mg over 60 minutes. Pedia - Venous catheter occlusion, Central, 30 kg of weight or greater) 2 mg/ 2 mL instilled into occluded catheter; up to 2 doses may be used, separated by 120 minutes. CONTRA-INDICATIONS: active internal bleeding, hypersensitivity to alteplase, severe uncontrolled hypertension, recent intracranial or intraspinal surgery or trauma, intracranial neoplasm, arteriovenous malformation, or aneurysm known bleeding diathesis, history of cerebrovascular accident, evidence of intracranial hemorrhage, suspicion of subarachnoid hemorrhage, history of intracranial hemorrhage, seizure at the onset of stroke, platelet count less than 100,000/mm(3), administration of heparin with 48 hours preceding the onset of stroke and have an elevated activated partial thromboplastin time at presentation ADVERSE EFFECTS: Cardiac dysrhythmia, Reperfusion, Cholesterol embolus syndrome, Gastrointestinal hemorrhage, After catheter clearance, Immune hypersensitivity reaction, Intracranial hemorrhage, Sepsis, After catheter clearance, Venous thrombosis, After catheter clearance THERAPEUTIC USE: Antacids DOSE: Adult - Oral doses are 15 to 30 mL or 250 mg to 1.5 g 4 times a day depending on the formula; pediatric oral doses are 0.5 to 5 mL or 150 to 250 mg/kg/day in 4 to 6 divided doses. Pedia – 0.5mL/kg are recommended for infants with reflux disease. Best results, as determined by serial intragastric pH monitoring, obtained when antacid was given prior to and after formula feedings CONTRA-INDICATIONS: Antacids are contraindicated in patients with a history of hypersensitivity to the components of the product. THERAPEUTIC USE: Amino Ester, Anesthetic, Local DOSE: Adult – direct application: apply 0.25% or 0.5% solution to larynx, trachea, or esophagus (add 0.06mL of epinephrine to each mL of anesthetic solution minor surgical procedures, instill 1-2 drops of 0.5% ophthalmic solution into eyes every 5-10 min for 1-3 instillations. Pedia - not FDA approved in pediatric patients CONTRA-INDICATIONS: hypersensitivity to tetracaine hydrochloride/ester-type anesthetics or aminobenzoic acid or its derivatives, topical application to inflamed/infected tissue, when spinal anesthesia contraindicated. ADVERSE EFFECTS: Apnea, Injection or excessive systemic absorption of topical Cardiac arrest, Injection or excessive systemic absorption of topical, Central nervous system finding, Depression or excitation, injection or excessive systemic absorption of topical, Contact dermatitis, Topical, Drug-induced keratoconjunctivitis (Severe), Ophthalmic THERAPEUTIC USE: Aminoglycoside Antibiotic DOSE: Adult – 15mg/kg/day IV/IM divided every 8 to 12 hr; depending on type and severity of infection; total daily dose should not exceed 15mg/kg/day; for heavier patients the total daily dose should not exceed 1.5g/day. Pedia – (neonates) 10mg/kg IV/IM loading dose followed with 7.5mg/kg IV/IM every 12 hr; total daily dose should not exceed 15 mg/kg/day; (infants and children) 15 mg/kg/day IV/IM divided every 8 to 12 hrs; total daily dose should not exceed 15 mg/kg/day ADVERSE EFFECT: Nephrotoxicity, Neuromuscular blockade finding, Ototoxicity, Respiratory tract paralysis, Concomitant anesthesia, muscle relaxants THERAPEUTIC USE: Diuresis DOSE: Diuresis: 1 tab ORALLY daily, may increase to 2 tabs ORALLY daily CONTRA-INDICATIONS: antikaliuretic therapy (potassium-conserving agents or supplementation), hypersensitivity to amiloride, HCTZ or sulfonamides, hyperkalemia impaired renal function (Cr over 1.5 mg/100 mL or BUN over 30 mg/100 mL) ADVERSE EFFECTS: Dermatologic: Erythema multiforme, Rash (3-8%), Scaling eczema (less than 1%), Endocrine metabolic: Hyperkalemia (1-2%), Gastrointestinal: Loss of appetite (3-8%), Nausea (3-8%), Neurologic: Asthenia (3-8%), Dizziness (3-8%), Headache (3-8%) 3 PRODUCT DESCRIPTION TRADE NAME Aminophyllin DOSAGE FORM/STRENGTH Injection 250mg Amiodarone Cordarone Injection 150mg Susp. 5mg/mL Tablet 200mg Amitriptyline HCl Tryptizol Tablet 10mg Tablet 25mg Amlodipine Amoxycillin Amlor Lowvasc Amoxil Amoxydar Flemoxin Hiconcil Hymox Julphamox Ospamox Amoclan Amoxycillin / Clavulanic Acid Augmentin Klavox Tablet 5mg Capsule 500mg Drops 100mg Susp.250mg/5mL Injection 600mg Injection 1200mg Susp. 156mg/5mL Susp. 457mg/5mL Tablet 375mg Tablet 625mg PRODUCT INFORMATION THERAPEUTIC USE: Asthma, chronic obstructive pulmonary disease DOSE: Adult - Asthma: aminophylline dose equals theophylline dose divided by 0.8; Asthma: IV, loading dose for acute bronchodilation, theophylline 4.6mg/kg (aminophylline 5.7mg/kg) of ideal body weight IV over 30 min. Pedia – Asthma IV, loading dose for acute bronchodilation, theophylline 4.6 mg/kg (aminophylline 5.7 mg/kg) of lean (ideal) body weight IV over 30 min. ADVERSE EFFECTS: Cardiovascular: Atrial fibrillation, Bradyarrhythmia, Too quick Administration, Cardiac arrest, Tachyarrhythmia, Dermatologic: Erythroderma Gastrointestinal: Necrotizing enterocolitis in fetus or newborn, Immunologic: Immune hypersensitivity reaction, Neurologic: Intracranial hemorrhage, Seizure THERAPEUTIC USE: Antiarrhythmic, Group III, Benzofuran DOSE: Adult – Advanced cardiac life support 300mg IV/IO by rapid infusion; if necessary, a supplementary dose of 150mg IV/IO by rapid infusion may be considered; MAX daily dose 2.2 g;Supraventricular arrhythmia – IV/ORAL regimen; 300mg IV for 1 hr, then 20 mg/kg for 24 hrs, then 600 mg ORALLY daily for 1 wk, then 400 mg ORALLY daily Pedia – safety and efficacy have not been established in children;Advanced cardiac life support perfusing tachycardias, 5 mg/kg IV over 2060 min; repeat up to MAX daily dose 15 mg/kg; MAX single dose 300 mg CONTRA-INDICATIONS: Cardiogenic shock, Hypersensitivity to amiodarone or to any of its components, including iodine,Second or third degree AV block, Severe sinus bradycardia, Severe sinus node dysfunction ADVERSE EFFECTS: Cardiac dysrhythmia, Congestive heart failure, Disorder of thyroid gland, Hepatotoxicity, Immune hypersensitivity reaction, Pulmonary toxicity, Shock, Stevens-Johnson syndrome, Thrombocytopenia THERAPEUTIC USE: Antidepressant, Tricyclic, Urinary Enuresis Agent DOSE: Adult – Depression 1)outpatients: 75 mg ORALLY (divided into 1-3 doses per day); may increase up to a max of 150 mg/day 2)inpatients: 100 mg ORALLY (divided into 1-3 doses per day); may increase up to a max of 300 mg/day 3)maintenance: 50-100 mg ORALLY at bedtime 4) 20-30 mg IM 4 times a day; switch to ORAL dosing as soon as possible. Pedia – Safety and effectiveness in children below 12 years have not been established CONTRA-INDICATIONS: co-administration with cisapride; may cause QT interval prolongation and increase the risk of arrhythmia, coadministration with a MAOI or use within 14 days of discontinuing a MAOI; may cause serious reactions (eg, hyperpyretic crisis, severe convulsions, death) hypersensitivity to amitriptyline hydrochloride myocardial infarction (MI), during the acute recovery period ADVERSE EFFECTS: Agranulocytosis, Aplastic anemia, Atrioventricular conduction pattern – finding, Cardiac dysrhythmia, Cerebrovascular accident, Decreased liver function, Depression, worsening, Drug-induced eosinophilia, Hypertension, Jaundice, Leukopenia, Myocardial infarction, Orthostatic hypotension, Pancytopenia, Purpuric disorder, Seizure, Suicidal thoughts, Suicide, Syncope,Thrombocytopenia THERAPEUTIC USE: Antianginal, Antihypertensive, Calcium Channel Blocker, Cardiovascular Agent, Dihydropyridine DOSE: Adult – Hypertension initial, 5 mg ORALLY once daily; maintenance 5-10 mg ORALLY once daily; Stable angina 5-10 mg ORALLY once daily. Pedia – safety and efficacy not established in pediatric patients younger than 6 years of age, Hypertension, 6 to 17 years of age: 2.5-5 mg ORALLY once daily CONTRA-INDICATIONS: Hypersensitivity to amlodipine ADVERSE EFFECTS: Angina, Cardiac dysrhythmia, Myocardial infarction THERAPEUTIC USE: Antibiotic Penicillin, Amino-penicillin DOSE: Adult - Infection of skin &/or subcutaneous tissue mild to moderate infections, 500 mg ORALLY every 12 hr or 250 mg ORALLY every 8 hr oral capsules, oral tablets, chewable oral tablets, oral suspension, severe infections, 875 mg ORALLY every 12hr or 500 mg ORALLY every 8 hr. Pedia – 50 mg/kg ORALLY 1 hr prior to procedure; maximum 2 g/dose. CONTRA-INDICATIONS: Hypersensitivity to penicillins ADVERSE EFFECTS: Immune hypersensitivity reaction THERAPEUTIC USE: Antibiotic DOSE: Adult – Infection of skin &/or subcutaneous tissue a)mild to moderate infections, 500 mg ORALLY every 12 hr or 250 mg ORALLY every 8 hr b) severe infections, 875 mg ORALLY every 12 hr or 500 mg ORALLY every 8 hr. Pedia – (125mg/5mL) infants less than 3 months of age, 30 mg/kg/day ORALLY divided every 12 hr; (200 mg and 400 mg/5mL, 200 mg and 400 mg chewable tab) 3 months and older and under 40 kg, 25-45 mg/kg/day ORALLY divided every 12 hr; depending on type and severity of infection; (125 mg and 250 mg/5mL, 125 mg and 250 mg chewable tab) 3 months and older and under 40 kg, 20-40 mg/kg/day ORALLY divided every 8 hr; depending on type and severity of infection CONTRA-INDICATIONS: hypersensitivity to penicillins, clavulanic acid, history of Augmentin-associated jaundice/hepatic dysfunction 4 PRODUCT DESCRIPTION Amphotericin Ampicillin Amylase/Lipase/Protease /Dimethylpolysiloxane/ Pancreatin Antazoline/Naphazolin Benzethonium TRADE NAME Abelect Fungizone Epicocillin Pentrexyl Pankreoflat Noscam DOSAGE FORM/STRENGTH Injection 50mg Lipid Complex – Injection 100mg Injection 500mg Injection 1gram PRODUCT INFORMATION ADVERSE EFFECTS: Hepatotoxicity THERAPEUTIC USE: Antifungal, Antiprotozoal, Polyene DOSE: Adult test dose: 1mg in 20mL D5W IV over 20-30 min; American mucocutaneous leishmaniasis 0.25-1mg/kg/day IV over 2-6 hours. Pedia test dose: 1mg in 20mL D5W IV over 20-30 min; INTRATHECAL neonatal doses have ranged from 0.5mg/day in 2mL of D5W to 0.6 mg/day in 0.5mL of D5W (total doses were 0.15 mg to 8.6 mg); doses of 0.125 to 0.25 mg have been administered to children via an Ommaya reservoir. ADVERSE EFFECTS: Anaphylaxis, Anemia, Blurred vision, Cardiac dysrhythmia, Diplopia, Hypokalemia, Hypotension, Nephrotoxicity, Seizure, Tachypnea, Thrombocytopenia, Thrombophlebitis THERAPEUTIC USE: Antibiotic, Penicillin, Aminopenicillin DOSE: Adult – Respiratory tract infection 250 mg ORALLY every 6 hr; 250-500 mg IV/IM every 6 hr. Pedia – Respiratory tract infection 50 mg/kg/day ORALLY divided every 6 hr CONTRA-INDICATIONS: Hypersensitivity to ampicillin products/penicillins ADVERSE EFFECTS: Immune hypersensitivity reaction Tablet THERAPEUTIC USE: Digestant, Enzyme DOSE: Adult – Exocrine pancreatic insufficiency 2 tablets (1000 mg) ORALLY with each meal and 2 tablets (1000 mg) taken with food eaten between meals. Pedia – safety and effectiveness in children have not been established CONTRA-INDICATIONS: Hypersensitivity to pancreatin or to pork protein Nasal Drops THERAPEUTIC USE: Allergic conjunctivitis DOSE: Adult – Allergic conjunctivitis: 1-2 drops 0.5% ophthalmic solution in each EYE up to 4 times daily (with 0.05% naphazoline). Pedia – Not FDA approved in children under 6 y of age; Allergic conjunctivitis: (6 y and older) 1-2 drops 0.5% ophthalmic solution in each EYE up to 4 times daily (with 0.05% naphazoline) CONTRA-INDICATIONS: concurrent use of contact lens, narrow angle glaucoma ADVERSE EFFECTS: Ophthalmic: Excessive tear production, Transient, Intraocular pressure finding, Increase or decrease, Mydriasis, Pain in eye, Transient Antazoline/Tetryzoline Spersallerg Eye Drops Anti-Haemophilic Factor IX Octanine F Injection 500iu Anti-Haemophilic Factor VII Novoseven Injection 1.2mg Anti-Haemophilic Factor VIII Octavi Injection 250iu Injection 500iu THERAPEUTIC USE: Inflammatory conditions of the conjunctiva of allergic origin, especially hay fever and vernal conjunctivitis, and in drug hypersensitivity reactions, excessive lacrimal secretion and stenosis of the lacrimal canal DOSE: Adult – Acute cases 1drop every three hours; Protracted treatment, 1drop 2-3times daily is sufficient. In severe cases apply hourly. Pedia – only 1-2 drops per day. THERAPEUTIC USE: Antihemophilic Agent, Hemostatic DOSE: Adult – Hemophilia B – Hemorrhage; number of Factor IX IU required = body weight (kg) times desired Factor IX increase (%) times 1 IU/kg; mild hemorrhage (20% to 30% of normal Factor IX level), 20-30 IU/kg IV twice daily until hemorrhage stops and healing is achieved (1 to 2 days). Pedia – Hemophilia B – Hemorrhage; dose required = body weight (kg) times desired Factor IX increase (%) times 1 IU/kg CONTRA-INDICATIONS: Hypersensitivity to hamster protein, Disseminated intravascular coagulation, fibrinolysis, Hypersensitivity to mouse proteins THERAPEUTIC USE: Antihemophilic Agent, Hemostatic DOSE: Adult & Pedia – Bleeding; Prophylaxis – Factor VII deficiency – Procedure 15-30 mcg/kg IV bolus over 2-5 minutes, every 4-6 hours until hemostasis achieved; adjust dose and frequency as necessary. CONTRA-INDICATIONS: Hypersensitivity to mouse, hamster, or bovine proteins, hypersensitivity to recombinant coagulation factor VIIa or any of its components ADVERSE EFFECTS: Acute renal failure, Anaphylaxis, Arterial thromboembolism, Bleeding, Blood coagulation disorder, Cerebral artery occlusion, Cerebral ischemia, Disseminated intravascular coagulation, Ischemic heart disease, acute, Myocardial infarction, Pulmonary embolism, Supraventricular tachycardia, Venous thromboembolism THERAPEUTIC USE: Antihemophilic Agent DOSE: Adult & Pedia; Bleeding – Hemophilia A – dosage calculation: body weight (kg) X factor VIII activity increase desired (%) divided by 2% per international unit per kg = dose required (international units). CONTRA-INDICATIONS: History of anaphylactic or severe systemic response to antihemophilic factor VIII, Hypersensitivity to antihemophilic factor VIII ADVERSE EFFECTS: Hemolytic anemia, Hyperfibrinogenemia, Immune hypersensitivity reaction, Pulmonary thromboembolism, Thromboembolic disorder, Viral disease, Transmission 5 PRODUCT DESCRIPTION TRADE NAME Anti-Rho(D) Immunoglobulin Rhesonativ Apraclonidine Iopidine Aprotinin Trasylol Artificial Tears DOSAGE FORM/STRENGTH Inj. 250mcg Eye Drops 5mg/mL Inj. 500,000u Eye Drops Ascorbic Acid + Ca Sandoz Ca-C Ca-Sandoz Cal-C-Vita Calcitec Tablet Asparaginase Erwinase Inj. 10,000u Cardol Atenolol Hypoten Tenormin Inj. 5mg/10mL Tablet 50mg Tablet 100mg PRODUCT INFORMATION THERAPEUTIC USE: Immune Serum DOSE: Adult & Pedia – Abortion, Isoimmuinization affecting pregnancy, abortion or other obstetric condition – Rhesus isoimmunization affecting pregnancy, Sensitization of Rho(D) negative females to Rho(D) positive blood; Prophylaxis a)IV or IM; abortion, amniocentesis after 34 weeks gestation, or any other manipulation late in pregnancy associated with increased risk of Rh isoimmunization, 600 IU (120 mcg) IM OR IV immediately b)IV or IM; threatened abortion, amniocentesis before 34 weeks gestation, or chorionic villus sampling, 1500 IU (300 mcg) ( IM OR IV immediately, repeat every 12 weeks for duration of pregnancy. CONTRA-INDICATIONS: Anaphylaxis or severe systemic reaction to human globulin, IgA deficiency; potential for IgA antibody development and anaphylactic reactions, infants; do not administer to infants when used for the suppression of Rh isoimmunization ADVERSE EFFECTS: Acute renal failure, Anaphylaxis, Intravascular hemolysis THERAPEUTIC USE: Alpha-2 Adrenergic Agonist, Anti-glaucoma DOSE: Adult - Laser surgery – Ocular hypertension, Postsurgical; Treatment and Prophylaxis – 1 drop (1% solution) in operative eye(s) 1 h before surgery and 1 drop immediately upon completion of surgery; Raised intraocular pressure; Adjunct 1 to 2 drops (0.5% solution) instilled to affected eye(s) 3 times daily. Pedia – safety and efficacy have not been determined in pediatric patients CONTRA-INDICATIONS: hypersensitivity to apraclonidine or clonidine, patients receiving MAO inhibitors ADVERSE EFFECTS: Cardiac dysrhythmia, Immune hypersensitivity reaction, Peripheral edema THERAPEUTIC USE: Hemostatic DOSE: Adult – Cardiopulmonary bypass operation, At increased risk for blood loss and blood transfusion – Hemorrhage, Perioperative; Prophylaxis-initial dose: 10,000 kallikrein inhibitory units IV (10 minutes prior to the loading dose); loading dose: 1 million to 2 million kallikrein inhibitory units IV (over 20 to 30 minutes and prior to sternotomy). Pedia – safety and efficacy not established in pediatric patients CONTRA-INDICATIONS: hypersensitivity to aprotinin or any product component, known or suspected previous aprotinin exposure (including aprotinin containing fibrin sealant) within the last 12 months; risk of anaphylactic, and potentially fatal reactions, even among patients who tolerate an initial test dose ADVERSE EFFECTS: Anaphylaxis, Cerebral artery occlusion, Heart failure, Myocardial infarction, Renal failure, Shock, Thrombotic disorder THERAPEUTIC USE: Diagnostic Agent, Lubricant, Surgical Aid, Ocular DOSE: Adult & Pedia – Xerophthalmia instill 1 drop 0.3% to 1% OPHTHALMIC solution into affected eye(s) 3 to 4 times daily, as needed CONTRA-INDICATIONS: Hypersensitivity to hypromellose THERAPEUTIC USE: Calcium Supplement, Nutritive Agent, Urinary Acidifier, Vitamin Combination, Adult Formula DOSE: Adult – mild to moderate deficiency, 100 to 250 mg IM/IV/SC or ORALLY once or twice daily. Pedia - Ascorbic acid deficiency 100 mg 3 times/day IM/IV/SC or ORALLY for 1 wk, then 100 mg daily for several wk until tissue saturation is reached; recommended dietary allowance, age 0 to 6 months, 40 mg/day; 7 to 12 months, 50 mg/day; 1 to 3 y, 15 mg/day; 4 to 8 y, 25 mg/day; 9 to 13 y, 45 mg/day; males 14 to 18 y, 75 mg/day; females 14 to 18 mg/day 65 mg/day CONTRA-INDICATIONS: Hypersensitivity to any component of the preparation THERAPEUTIC USE: Antineoplastic Agent DOSE: Adult & Pedia – 1)desensitization therapy, administer 1 International Unit IV and double the dose every 10 minutes, provided no reaction has occurred, until the accumulated total amount equals the planned dose for that day 2)skin test, prepare by reconstituting the contents of a 10,000 International Units vial with 5 mL of diluent. From this solution (2,000 International Units/mL) 0.1 mL is withdrawn and injected into another vial containing 9.9 mL diluent, giving a skin test solution of approximately 20 International Units/mL. For the intradermal skin test, 0.1 mL of this solution (approximately 2 International Units) is utilized. CONTRA-INDICATIONS: acute hemorrhagic pancreatitis, history of pancreatitis, pancreatitis, hypersensitivity reactions/anaphylaxis ADVERSE EFFECTS: Acute hemorrhagic pancreatitis, Acute renal failure, Anaphylaxis, Body temperature above normal, Hyperglycemia, Hypofibrinogenemia, Factors V, VII, VIII & IX, Pancreatitis, Pharyngitis, Phlebitis, Pseudocyst of pancreas, Rash, Respiratory distress, Urticaria THERAPEUTIC USE: Antianginal, Antiarrhythmic, Group II, Antihypertensive, Antimigraine, Beta-Adrenergic Blocker, Cardioselective, Cardiovascular Agent DOSE: Adult – Acute myocardial infarction – 5 mg IV over 5 min, give second 5 mg IV dose 10 min later; if IV dose was tolerated, begin 50 mg ORALLY 10 min after last IV dose, then 50 mg ORALLY 12 h later, then 100 mg ORALLY once daily or 50 mg ORALLY twice daily for 6 to 9 further days; Angina pectoris, chronic 50 mg ORALLY once daily; if inadequate response after 1 week, increase to 100 mg ORALLY once daily; maximum dose, 200 mg ORALLY once daily. Pedia – Not FDA-approved in children Hypertension – initial, 0.5 to 0.1 mg/kg/day ORALLY in 1 to 2 divided doses; MAX 2 mg/kg/day ORALLY up to 100 mg/day in 1 to 2 divided doses CONTRA-INDICATIONS: cardiogenic shock, hypersensitivity to atenolol, overt cardiac failure, second and third degree AV block, severe sinus bradycardia, ADVERSE EFFECTS: Lupus erythematosus 6 PRODUCT DESCRIPTION Atosiban Acetate TRADE NAME Tractocile DOSAGE FORM/STRENGTH Inj. 37.5mg/5mL Inj. 6.75mg/0.9mL Eye Drops 0.5% Eye Oint. 1% Inj. 0.5mg/mL Syr. 0.1mg/mL Atropine Sulphate Azathioprine Imuran Tablet 50mg Aztreonam Azactam Injection 1gram Baclofen Lioresal Syrup 5mg/mL Tablet 10mg Balanced Salt BSS Eye Solution BCG Live Attenuated Mycobacterum Tuberculosis Injection PRODUCT INFORMATION THERAPEUTIC USE: Effective in the treatment of preterm labor, Intravenous atosiban effectively inhibits uterine contractions DOSE: Preterm labor – Optimal doses have not been established. Atosiban is given via a continuous intravenous infusion. An initial intravenous bolus is recommended to shorten the time to cessation of uterine contractions. For women with preterm labor and intact membranes, an effective intravenous (IV) dosing regimen has begun with a 6.75 milligram bolus, followed immediately by infusion of 300 micrograms/minute for 3 hours and then a 100 micrograms/minute infusion CONTRA-INDICATIONS: hypersensitivity to atosiban, Preeclampsia or eclampsia, suspected chorioamnionitis, bruption placentae, undiagnosed vaginal bleeding, multiple gestation, intrauterine fetal distress or fetal death THERAPEUTIC USE: Anesthetic Adjunct, Antimuscarinic, Cholinergic Antagonist, Gastrointestinal Agent, Mydriatic-Cycloplegic, Nerve Gas Antidote, Urinary Antispasmodic DOSE: Adult – Bradyarrhythmia, acute symptomatic, 0.5 mg IV every 3 to 5 min to MAX total dose of 3 mg; Cardiac dysrhythmia, Increased vagal activity during intra-abdominal surgical traction - General anesthesia, 0.4 to 0.6 mg IV; Cycloplegic refraction - 1 drop of 1% or 2% ophthalmic solution instilled in eye(s) up to 3 times a day. Pedia – Cardiac dysrhythmia, Increased vagal activity during intra-abdominal surgical traction - General anesthesia - a)up to 12 y, 0.1 to 0.6 mg IV b)12 y and older, 0.4 to 0.6 mg IV c)with cyclopropane anesthetic, less than 0.4 mg IV slowly; Amblyopia - 1 drop of 1% ophthalmic solution instilled in eye(s) once daily CONTRA-INDICATIONS: Hypersensitivity to atropine or anticholinergics, Narrow-angle glaucoma, Reflux esophagitis, Obstructive gastrointestinal disease, Ulcerative colitis or toxic megacolon, Obstructive uropathy, Unstable cardiovascular status in acute hemorrhage or thyrotoxicosis, Paralytic ileus or intestinal atony, Myasthenia gravis ADVERSE EFFECTS: Cardiac dysrhythmia, Coma, Immune hypersensitivity reaction, Raised intraocular pressure, Respiratory depression THERAPEUTIC USE: Antimetabolite, Antirheumatic, Cytotoxic, Gastrointestinal Agent DOSE: Adult – Inflammatory bowel disease 2-3 mg/kg/day ORALLY; maintenance, 1 to 2.5 mg/kg/day ORALLY OR IV as single dose or divided twice daily; may lower dose 0.5 mg/kg/day every 4 weeks until lowest effective dose is reached. Pedia – safety & efficacy not established in pediatric patients CONTRA-INDICATIONS: history of treatment with alkylating agents, hypersensitivity to azathioprine, pregnancy ADVERSE EFFECTS: Cancer, Hepatotoxicity, Infectious disease, In renal transplant patients, Leukopenia, Megaloblastic anemia, Pancreatitis, Thrombocytopenia THERAPEUTIC USE: Antibiotic, Monobactam DOSE: Adult - Infection of skin &/or subcutaneous tissue (moderately severe systemic infections) 1 or 2 g IV every 8 or 12 h (MAX dose, 8 g/day) Pedia – Endometritis (mild to moderate infections) age 9 months and older, 30 mg/kg IV every 8 h (MAX dose, 120 mg/kg/day) ADVERESE EFFECTS: Anaphylaxis, Hepatotoxicity, Neutropenia, Pancytopenia, Pseudomembranous enterocolitis, Thrombophlebitis THERAPEUTIC USE: Analgesic, Gamma Aminobutyric Acid (class), Skeletal Muscle Relaxant, Centrally Acting DOSE: Adult – Spasticity 5mg ORALLY 3 times a day; may increase dosage by 15 mg/day increments every 3 days to a MAX dose of 80 mg/day (3 to 4 divided doses) Pedia – Safety and effectiveness of oral baclofen use in children under 12 years of age have not been established. Spasticity a)(2 to 7 y old) 10 to 15 mg/day ORALLY (2 to 3 divided doses); may increase by 5 to 15 mg/day increments every 3 days to a MAX dose of 40 mg/day (3 to 4 divided doses) b)(8 y and older), 10 to 15 mg/day ORALLY (2 to 3 divided doses); may increase by 5 to 15 mg/day increments every 3 days to a MAX dose of 60 mg/day (3 to 4 divided doses) ADVERSE EFFECTS: Aseptic meningitis, with intrathecal administration, Coma, Death, abrupt withdrawal, Seizure THERAPEUTIC USE: For irrigation during various surgical procedures of the eyes, ears, nose and/or throat. DOSE: This ocular irrigating solution should be used according to standard format for each surgical procedure. ADVERSE EFFECTS: When the corneal endothelium is abnormal, irrigation or any other trauma may result in bullous keratopathy. Postoperative inflammatory reactions as well as incidents of corneal edema and corneal decompensation have been reported THERAPEUTIC USE: Vaccine DOSE: Adult - Tuberculosis; Prophylaxis 0.2-0.3 mL PERCUTANEOUSLY x 1. Pedia –Tuberculosis; Prophylaxis a)1 month of age and older, 0.2-0.3 mL PERCUTANEOUSLY x 1; b)less than 1 month of age, one-half of adult dose (by reconstituting vial with 2 mL of sterile water) CONTRA-INDICATIONS: immunocompromised status including HIV infection, urinary tract infection or hematuria, acute febrile illness, 7 to 14 days following biopsy, transurethral resection, or traumatic catheterization; increased risk for systemic BCG infection, active tuberculosis, hypersensitivity to BCG products ADVERSE EFFECTS: Caseous lesion, Puncture site, percutaneous, Disseminated mycobacteriosis, Intravesical and percutaneous, Infection due to Mycobacterium bovis, Intravesical and percutaneous, Injection site ulcer, Percutaneous, Purulent discharge from wound, Suppurative lymphadenopathy, With draining sinuses, percutaneous 7 PRODUCT DESCRIPTION TRADE NAME Beclomethasone Dipropionate Becloforte Beconase Becotide Benzathine Penicillin Retarpen DOSAGE FORM/STRENGTH Inhaler 50mcg Nasal Spray 50mcg Inj. 1.2million units Benzoic Acid Powder Benzoin Tincture Solution Benztropine Mesylate Cogentin Inj. 1mg/mL Benzyl Penicillin Sodium Specillin Inj. 1million unit Beractant Survanta Suspension Betahistine Dihydrochloride Betaserc Tablet 8mg Betamethasone + Cinchocaine Supraproct-S Suppository Betamethasone Dipropionate Betasone Cream 0.1% Betnovate PRODUCT INFORMATION THERAPEUTIC USE: Adrenal Glucocorticoid, Anti-Inflammatory DOSE: Adult - Asthma, maintenance treatment and prophylaxis Oral Inhalation, previously treated with bronchodilators alone, initial, 40 to 80 mcg twice a day; MAX 320 mcg twice daily. Pedia - Safety and efficacy not established in children under age 5; Asthma, maintenance treatment and prophylaxis Oral Inhalation, ages 5 to 11 yr, 40 mcg twice a day; MAX 80 mcg twice a day CONTRA-INDICATIONS: Acute asthma exacerbations, hypersensitivity to beclomethasone, status asthmaticus ADVERSE EFFECTS: Cataract, Glaucoma, Osteoporosis, Long-term treatment, Secondary hypocortisolism THERAPEUTIC USE: Treatment & prophylaxis of infections caused by organisms with high penicillin sensitivity DOSE: Adult – 1 to 2dose of Retarpen 1.2mu at intervals of 4weeks. Pedia – under 12years old 1dose of Retarpen 0.6mu at intervals of 14 days. CONTRA-INDICATIONS: Hypersensitivity to to Penicillins, cephalosphorins. ADVERSE EFFECTS: Stomatitis, glossitis, neuropathy, nephropathy, urticaria. THERAPEUTIC USE: Benzoic Acid (class), Scabicide DOSE: Adult – Apply benzoic acid and salicylic acid ointment to affected area 1 or 2 times daily. Duration of therapy may be weeks to months depending on the infection being treated. Pedia - Benzyl benzoate is diluted with water to 12.5% in children and 8.3% in infants prior to application. It is applied topically to the entire body from the neck down. The lotion should be left on skin for 24 hours, then washed off THERAPEUTIC USE: Aromatic Inhalation, Inhalations are used for the relief of nasal obstruction in acute rhinitis or sinusitis. DOSE: Add one teaspoonful to a pint of hot, not boiling water and inhale the vapour. The use of strong aromatic decongestants (applied as rub or to pillows) is not advised for infants under the age of 3 months. THERAPEUTIC USE: Anticholinergic, Antiparkinsonian DOSE: Adult & Pedia – Extrapyramidal disease – Medication-induced movement disorder, 1 to 4 mg IM/IV/ORAL once or twice a day; Parkinsonism 1 to 2 mg/day IM/IV/ORAL (range 0.5 to 6 mg/day) CONTRA-INDICATIONS: hypersensitivity to benztropine or any component, less than age 3 years ADVERSE EFFECTS: Confusion, Drug-induced psychosis, Heat stroke, Hyperpyrexia, Paralytic ileus, Raised intraocular pressure THERAPEUTIC USE: Antibiotic, Penicillin, Natural DOSE: Adult - Bacteremia, Gram-negative usual doses range from 5 to 24 million units/day IV in divided doses every 4 to 6 h. Pedia Meningitis (Pneumococcus, Neisseria eningitides/Meningococcus) 250,000 units/kg/day IV/IM in equally divided doses every 4 h for 7 to 14 days; MAX dose, 12 to 20 million units/day) CONTRA-INDICATIONS: History of penicillin hypersensitivity ADVERSE EFFECTS: Hemolytic anemia, With large IV doses, Hyperkalemia, High doses, decreased renal function, Immune hypersensitivity reaction, Interstitial nephritis, With rash, fever, eosinophilia, Seizure, Patients with renal failure, infants, elderly, meningitis, history of seizures THERAPEUTIC USE: Lung Surfactant DOSE: Pedia – Respiratory distress syndrome in the newborn first dose; 100 mg/kg (4mL SurvantaI/kg) INTRATRACHEALLY; slowly inject one quarter of dose (over 2 to 3 seconds) via catheter into endotracheal tube, remove catheter and manually ventilate at 60 breaths/min for 30 seconds or until stable, reattach catheter and instill next 3 quarter doses using the same procedure; do not suction infant for 1 hour after dosing unless significant airway obstruction occurs; during rescue strategy use mechanical ventilator rather than manual ventilation CONTRA-INDICATIONS: None known. ADVERSE EFFECTS: Blocked endotracheal tube, Bradyarrhythmia, Transient, Desaturation of blood, Reflux, Endotracheal tube THERAPEUTIC USE: Cerebrovascular disease, vertigo, retinal detachment, sensorineural hearing loss DOSE: Adult – In MENIERE'S DISEASE and other audiovestibular disorders, betahistine has been given in doses of 8 to 16 milligrams orally 3 times daily (total, 24 to 48 milligrams/day). It is recommended that the drug be taken with meals; Oral doses of 8 to 16 milligrams 3 times daily have been used in patients with cerebrovascular disease THERAPEUTIC USE: External & internal haemorrhoids. DOSE: Adult – 1suppository or a little smear of the ointment should be applied morning and evening until the acute symptoms have disappeared, can be reduced to one application per day or according to the instructions of the Doctor. CONTRA-INDICATIONS: Hypersensitivity to the preparation. THERAPEUTIC USE: Adrenal Glucocorticoid, Corticosteroid, Diagnostic Agent, Endocrine-Metabolic Agent, Immune Suppressant DOSE: Adult & Pedia (13years & older) – Topical (0.5% Lotion, Cream or Ointment), apply to affected area once or twice daily, Max 50 g/wk (ointment) or 50mL/wk (lotion) for no more than 2 wk CONTRA-INDICATIONS: hypersensitivity to betamethasone products, hypersensitivity to other corticosteroids, systemic fungal infections ADVERSE EFFECTS: Cataract, Cushing's syndrome, Glaucoma, Hyperglycemia, Osteoporosis, Primary adrenocortical insufficiency, Pulmonary tuberculosis 8 PRODUCT DESCRIPTION TRADE NAME DOSAGE FORM/STRENGTH Betaxolol Betoptic Eye Soln 0.5% Bevacizumab Avastin Inj. 100mg/4mL Bisacodyl Dulcolax Supp. 5mg Supp. 10mg Tablet 5mg Bisoprolol Fumarate Concor Tablet 2.5mg Tablet 5mg Tablet 10mg Bleomycin HCl Bleocina Injection 15mg Botulinum Toxin Botox Inj. 100units Bretylium Tosylate Bretylol Inj. 50mg/mL Bromazepam Lexotanil Tablet 3mg PRODUCT INFORMATION THERAPEUTIC USE: Antiglaucoma, Antihypertensive, Beta-Adrenergic Blocker, Cardioselective, Cardiovascular Agent DOSE: Adult & Pedia – Ocular hypertension & primary open angle glaucoma (0.25% suspension) 1 drop in the affected eye(s) twice daily; (0.5% solution) 1 to 2 drops in the affected eye(s) twice daily. Safety and efficacy not established in children for the 0.5% ophthalmic solution CONTRA-INDICATIONS: cardiogenic shock, hypersensitivity to betaxolol, overt cardiac failure, second and third degree AV block, severe sinus bradycardia ADVERSE EFFECTS: Anemia, Cardiac dysrhythmia, Heart failure, Myocardial infarction Thrombocytopenia THERAPEUTIC USE: Immunological Agent, Monoclonal Antibody DOSE: Adult - Metastatic breast cancer, HER2-negative disease, first-line therapy in combination with paclitaxel The dose of bevacizumab used in the phase III clinical trial was 10 milligrams/kilogram (mg/kg) intravenously on days 1 and 15 every 28 days. Paclitaxel was administered at a dose of 90 mg/square meter intravenously on days 1, 8, and 15, of the same 28-day cycle for a maximum of 18 cycles, or until disease progression or unacceptable toxicity. Pedia - the safety and effectiveness of bevacizumab in pediatric patients has not been established ADVERSE EFFECTS: Arterial thromboembolism, Asthenia, Bleeding, Complication of infusion (Severe), Congestive heart failure, Deep venous thrombosis, Diarrhea, Gastrointestinal hemorrhage, Gastrointestinal perforation, Hemoptysis, Hypertension, Hyponatremia, Impaired wound healing, Leukopenia, Nephrotic syndrome, Neutropenia, Pulmonary hemorrhage, Reversible posterior leukoencephalopathy syndrome, Thrombocytopenia, Thromboembolic disorder, Tracheoesophageal fistula, Venous thromboembolismWound dehiscence THERAPEUTIC USE: Laxative, Stimulant DOSE: Adult – use for more than 7 days is not recommended; Constipation 5-15 mg ORALLY once daily up to 30 mg/day10 mg suppository RECTALLY once daily. Preparation of bowel for procedure 10 to 15 mg ORALLY once daily up to 30 mg/day;10 mg suppository RECTALLY once daily. Pedia – use for more than 7 days is not recommended. Constipation (6-11 yrs) 5 mg ORALLY once daily; (6-11 yrs) 5 mg suppository RECTALLY once daily (under 6 yrs) consult physician CONTRA-INDICATIONS: appendicitis, intestinal obstruction, gastroenteritis ADVERSE EFFECTS: Atony of colon THERAPEUTIC USE: Antianginal, Antihypertensive, Beta-Adrenergic Blocker,, Cardioselective, Cardiovascular Agent DOSE: Adult – initial, 2.5-5 mg ORALLY once daily; maintenance, 2.5-20 mg ORALLY once daily; maximum dose, 40 mg ORALLY once daily. Pedia –safety and efficacy not established in children CONTRA-INDICATIONS: cardiogenic shock, hypersensitivity to bisoprolol, overt cardiac failure, second and third degree AV block, severe sinus bradycardia THERAPEUTIC USE: Antibiotic DOSE: Adult – Hodgkin's disease treatment, 10 to 20 units/m(2) (0.25 to 0.5 units/kg) IV/IM/SC once or twice weekly ; maintenance, following a 50% response, use a maintenance dose of 1 unit once daily or 5 units once weekly IV/IM. Pedia - safety and efficacy have not been established in pediatric patients; protocol-specific, adult doses have been used on a case-by-case basis CONTRA-INDICATIONS: hypersensitivity / idiosyncratic reactions to bleomycin products ADVERSE EFFECTS: Hepatotoxicity, Hypotension, Idiosyncratic drug effect, Myelosuppression, Nephrotoxicity, Pneumonitis, Possibly progressing to pulmonary fibrosis, Pulmonary fibrosis, Usually higher in elderly population and with > 400 units total dose, Vascular disorder, MI, CVA, Raynaud's, Wheezing THERAPEUTIC USE: Musculoskeletal Agent, Neuromuscular Blocker, Non-Depolarizing DOSE: Adult & Pedia – Blepharospasm 1.25-2.5 Units injected into orbicularis oculi muscle; 30 day cumulative MAX 200 Units. CONTRA-INDICATIONS: Hypersensitivity to botulinum A toxin products, Infection at the proposed injection site(s) ADVERSE EFFECTS: Anaphylaxis, Cardiac dysrhythmia, Hepatotoxicity, Hypertension, Myocardial infarction, Syncope, Strabismus patients THERAPEUTIC USE: Adrenergic Blocker, Antiarrhythmic, Group III DOSE: Adult – Ventricular arrhythmia; Treatment and Prophylaxis initial, 5-10 mg/kg IV bolus, may repeat up to MAX loading dose of 30 mg/kg IV; continuous suppression, 1-2 mg/min IV or 5-10 mg/kg over 8 min every 6 hr; 5-10 mg/kg IM, may repeat at 1-2 hr intervals, thereafter every 6-8 hr. Pedia – not FDA approved in children CONTRA-INDICATIONS: all contraindications are relative to the seriousness of the arrhythmia, digitalis-induced arrhythmias, hypersensitivity to bretylium ADVERSE EFFECTS: Cardiac dysrhythmia, Kidney disease THERAPEUTIC USE: Benzodiazepine, Short or Intermediate Acting, Sedative-Hypnotic DOSE: Adult – oral doses of 6 to 30 milligrams daily (single or divided doses) has proven effective in anxiety-neurosis. Usual doses for the treatment of anxiety range from 3 to 18 milligrams daily in divided doses. Single daily doses of 1.5 to 6 milligrams administered in the evening have produced good results in anxious patients CONTRA-INDICATIONS: Hypersensitivity to bromazepam, Narrow-angle glaucoma 9 PRODUCT DESCRIPTION Bromocriptine Mesylate Budesonide Bupivacaine HCl TRADE NAME DOSAGE FORM/STRENGTH Parlodel Tablet 2.5mg Entocort Enema 2mg/100mL Nebs. 0.5mg/mL Pulmicort Marcaine Marcaine Heavy Injection 0.25% Injection 0.5% Injection 0.75% Inj. Heavy 0.5% Busulphan Myleran Tablet 2mg Cabergoline Dostinex Tablet 0.5mg Calcipotriol Daivonex Cream 30grams Oint. 30grams PRODUCT INFORMATION THERAPEUTIC USE: Antiparkinsonian, Dopamine Agonist, Prolactin Secretion Inhibitor DOSE: Adult – Acromegaly initial, 1.25 to 2.5 mg ORALLY at bedtime; increase 1.25 to 2.5 mg every 3 to 7 days as needed; usual maintenance dose, 20 to 30 mg ORALLY daily; MAX 100 mg daily. Pedia –safety & effectiveness not established in pediatric patients age younger than 15 years; Prolactinoma a)(11 to 15 y old) 1.25 to 2.5 mg ORALLY daily, gradual dose adjustment to therapeutic response; usual dose range, 2.5 to 10 mg daily b)(16 y and older) initial, 1.25 to 2.5 mg ORALLY daily, increase dose by 2.5 mg every 2 to 7 days as needed until optimal therapeutic response achieved; usual dose range, 2.5 to 15 mg daily CONTRA-INDICATIONS: during postpartum period in women with history of coronary artery disease or other severe cardiovascular conditions unless withdrawal is considered medically contraindicated, hypersensitivity to bromocriptine products, sensitivity to ergot alkaloids, uncontrolled hypertension ADVERSE EFFECTS: Cerebral ischemia, Cerebrovascular accident, Confusion, Disorder of lower respiratory system, Pleuropulmonary, longterm use, Dyskinesia, Hallucinations, Myocardial infarction, Postpartum, Psychotic disorder, Seizure THERAPEUTIC USE: Adrenal Glucocorticoid, Anti-Inflammatory, Endocrine-Metabolic Agent DOSE: Adult – Asthma, Maintenance treatment Oral Inhalation, initial, 360 mcg twice daily, 180 mcg twice daily initially may be sufficient in some patients; MAX 720 mcg twice daily. Pedia – oral inhalation powder, not recommended for age 6 years or younger; Asthma, Maintenance treatment Oral Inhalation, (6 yr and older) initial, 180 mcg twice daily, 360 mcg twice daily may be required initially in some patients; MAX 360 mcg twice daily. CONTRA-INDICATIONS: hypersensitivity to budesonide products, status asthmaticus or other acute episodes of asthma ADVERSE EFFECTS:Cataract,Cushing's syndrome,Glaucoma, Secondary hypocortisolism THERAPEUTIC USE: Amino Amide, Anesthetic, Local DOSE: Adult – Administration of analgesic, Local 1)INTRAPLEURAL, 10-30 mL bolus of 0.25%, 0.375%, or 0.5% every 4-8 hr; 2) INTRAPLEURAL, continuous infusion 0.375% bupivacaine with epinephrine at 6 mL/hr after 20 mL loading dose. Pedia - administration in children under 12 years of age is not recommended; bupivacaine spinal with dextrose not recommended in children under 18 years of age; Administration of analgesic, Local INTRAPLEURAL, continuous infusion 0.25% bupivacaine with epinephrine at 0.5 mL/kg/hr CONTRA-INDICATIONS: hypersensitivity to bupivacaine products or to other amide-type anesthetics, local infection at the site of proposed lumbar puncture (spinal anesthesia), obstetrical paracervical block anesthesia, septicemia (spinal anesthesia), severe hemorrhage, severe hypotension or shock and arrhythmias, such as complete heart block, which severely restrict cardiac output (spinal anesthesia), sulfite allergy (epinephrine containing solutions only) ADVERSE EFFECTS: Bradyarrhythmia, CNS depression, Possibly proceeding to respiratory arrest, CNS stimulation, Possibly proceeding to convulsions, Hypotension, Up to 10% in spinal anesthesia, Respiratory arrest, Tinnitus, Ventricular arrhythmia THERAPEUTIC USE: Alkylating Agent, Antineoplastic Agent DOSE: Adult – Chronic myeloid leukemia, palliative, induction, 60 mcg/kg or 1.8 mg/m(2) ORALLY every day; usual dose is 4 to 8 mg; maintenance, 1 to 3 mg ORALLY every day. Pedia – Although ORAL busulfan is indicated for the treatment of chronic myelogenous leukemia in children, the safety and efficacy of IV busulfan in pediatric patients have not been determined; Chronic myeloid leukemia, palliative, induction, 60 mcg/kg or 1.8 mg/m(2) ORALLY every day; usual dose is 4 to 8 mg CONTRA-INDICATIONS: hypersensitivity to busulfan products, patients in whom a definitive diagnosis of chronic myelogenous leukemia has not been established ADVERSE EFFECTS: Amenorrhea, Aplastic anemia, Cardiac tamponade, Cataract, Complete atrioventricular block, Third degree, Disorder of adrenal gland, Addisonian-like Fibrosis of pericardium, Graft versus host disease, Hemorrhagic cystitis, Hypertension, Hypotension, Immune hypersensitivity reaction, Left heart failure, Leukemia, Male infertility, Myelosuppression, Leukopenia, thrombocytopenia, anemia, Neoplastic disease, Ovarian dysfunction, Pulmonary fibrosis, Seizure, Veno-occlusive disease of the liver THERAPEUTIC USE: Antiparkinsonian, Dopamine Agonist, Prolactin Secretion Inhibitor DOSE: Adult – Hyperprolactinemia initial, 0.25 mg ORALLY twice weekly, increase by 0.25 mg twice weekly at 4 wk intervals; MAX 1 mg twice weekly; Lactation suppression, Puerperal 1 mg ORALLY given within 24 to 27 h of delivery. Pedia – safety and efficacy not established in pediatric patients CONTRA-INDICATIONS: hypersensitivity to ergot derivatives, uncontrolled hypertension ADVERSE EFFECTS: Abdominal pain, Heart valve disorder, Orthostatic hypotension, Pleural effusion, Pulmonary fibrosis, Valvular regurgitation, Vertigo THERAPEUTIC USE: Antipsoriatic, Vitamin D3, Synthetic DOSE: Adult – Plaque psoriasis, cream; apply thin layer topically twice daily for up to 8 weeks; ointment; apply thin layer topically once or twice daily for up to 8 weeks. Pedia - safety and efficacy in children have not been established CONTRA-INDICATIONS: do not apply to face, hypercalcemia, hypersensitivity to calcipotriene or any ingredient, vitamin D toxicity 10 PRODUCT DESCRIPTION Calcitonin Calcium Carbonate TRADE NAME Miacalcic Caltrate DOSAGE FORM/STRENGTH Inj. 100units Inj. 200units Nasal Spray 100units Tablet 500mg Oscal Calcium Chloride Injection 10% Calcium Folinate (Ca Leucovorin) Capsule 15mg Inj.100mg/10mL Inj. 50mg/5mL Calcium Glubionate Ca-Sandoz Calcium Gluconate Syrup 1.2grams/5mL Injection 10% Calcium Polystyrene Sulphonate Calcium Resonium Powder Capecitabine Xeloda Tablet 500mg PRODUCT INFORMATION THERAPEUTIC USE: Hypercalcemia, Paget's disease, Postmenopausal osteoporosis DOSE: Adult – Hypercalcemia, initial, 4 international units/kg SUBQ or IM every 12 h; may increase after 1 to 2 days to 8 international units/kg every 12 h; may increase after another 2 days to a maximum of 8 international units/kg every 6 h; Paget's disease initial, 100 international units SUBQ or IM daily. Pedia – Safety and effectiveness not established in children CONTRA-INDICATIONS: hypersensitivity to synthetic calcitonin-salmon ADVERSE EFFECTS: Anaphylaxis, Anemia, Bronchospasm, Few cases, Cerebrovascular accident, Myocardial infarction, Thrombophlebitis THERAPEUTIC USE: Antacid, Calcium Regulator & Supplement, Dental Agent, Dermatological Agent, Hemostatic, Nutriceutical, Parenteral Mineral-Trace Mineral, Phosphate Binder DOSE: Adult – Calcium deficiency; Prophylaxis 500 mg (as elemental calcium) ORALLY 2 to 3 times daily with food. ADVERSE EFFECTS: Bradyarrhythmia, Hypercalciuria, Hypotension, Muscle weakness, Vasodilatation THERAPEUTIC USE: Antacid, Calcium Regulator & Supplement, Dental Agent, Dermatological Agent, Hemostatic, Nutriceutical, Parenteral Mineral-Trace Mineral, Phosphate Binder DOSE: Adult - Hypocalcemia, calcium chloride 500 mg to 1 g (5 to 10mL of a 10% solution) IV every 1 to 3 days depending on patient response; MAX rate, 1mL/min. CONTRA-INDICATIONS: digitalis toxicity, IM/SC injection of calcium chloride, ventricular fibrillation ADVERSE EFFECTS: Bradyarrhythmia, Cardiac arrest, With rapid IV injection, Cardiac dysrhythmia, Hypercalcemia, Hypercalciuria, Hypertension, Hypomagnesemia, Hypotension, Milk alkali syndrome, Muscle weakness, Vasodilatation THERAPEUTIC USE: Methotrexate Rescue, Nutritive Agent DOSE: Adult & Pedia – Antimetabolite overdose, Folic acid antagonist, 15 mg (10 mg/m(2)) IM/IV/ORAL every 6 hr until serum methotrexate (MTX) level below 0.01 micromolar (mcmol); if 24 hr serum creatinine 50% above baseline, 24 hr MTX level above 5 mcmol, OR 48 hr MTX level above 0.9 mcmol, increase dose to 100 mg/m(2) IV every 3 hr until serum MTX less than 0.01 mcmol. CONTRA-INDICATIONS: hypersensitivity to leucovorin, megaloblastic anemias due to vitamin B12 deficiency, pernicious anemias due to vitamin B12 deficiency ADVERSE EFFECTS: Allergy THERAPEUTIC USE: Antacid, Calcium Regulator & Supplement, Dental Agent, Dermatological Agent,, Hemostatic, Nutriceutical, Parenteral Mineral-Trace Mineral, Phosphate Binder DOSE: Adult – Calcium deficiency; Prophylaxis 500 mg (as elemental calcium) orally 2 to 3 times daily with food. ADVERSE EFFECTS: Bradyarrhythmia, Hypercalciuria, Hypotension, Muscle weakness, Vasodilatation THERAPEUTIC USE: Antacid, Calcium Regulator & Supplement, Dental Agent, Dermatological Agent, Hemostatic, Nutriceutical, Parenteral Mineral-Trace Mineral, Phosphate Binder DOSE: Adult – Allergic disorder,calcium gluconate 500 mg to 2 grams (10% solution) IV administered at a rate not to exceed 200 mg/min. Pedia – Hyperkalemia (children) calcium gluconate 200 to 500 mg (10% solution) IV at a rate not to exceed 200 mg/min; (infants) calcium gluconate up to 200 mg (10% solution) IV at a rate not to exceed 200 mg/min CONTRA-INDICATIONS: digitalis toxicity, IM/SC injection of calcium gluonate, ventricular fibrillation ADVERSE EFFECTS: Bradyarrhythmia, Cardiac arrest, With rapid IV injection, Cardiac dysrhythmia, Hypercalcemia, Hypercalciuria, Hypertension, Hypomagnesemia, Hypotension, Milk alkali syndrome, Muscle weakness, Vasodilatation THERAPEUTIC USE: Exchange Resin, Hyperkalemia DOSE: Adult – Hyperkalemia, 15 g ORALLY 1 to 4 times daily as a slurry in water or syrup; 30 to 50 g RECTALLY every 6 hr as a warm emulsion in 100 mL aqueous vehicle (sorbitol), retain 30-60 min and follow with a cleansing enema. Pedia – Hyperkalemia, 1 g/kg/dose ORALLY every 6 hr; do not use ORALLY in neonates;1 g/kg/dose RECTALLY every 2-6 hr CONTRA-INDICATIONS: hypersensitivity to sodium polystyrene sulfonate, hypokalemia, neonates with reduced GI motility (postoperatively or drug-induced), obstructive bowel disease, oral administration in neonates ADVERSE EFFECTS: Bronchopneumonia, Electrolytes abnormal, Fecal impaction, Rectal administration, Gastrointestinal necrosis, Colonic, Hypocalcemia, Hypokalemia THERAPEUTIC USE: Antimetabolite, Antineoplastic Agent DOSE: Adult – Colon cancer, Adjuvant therapy, Duke's Stage C, when treatment with a fluoropyrimidine alone is preferred 1,250 mg/m(2) ORALLY twice daily for 2 wks followed by 1 wk off, given as 3 wk cycles for a total of 8 cycles (6 mo). Pedia – safety and effectiveness in children have not been established CONTRA-INDICATIONS: dihydropyrimidine dehydrogenase (DPD) deficiency, hypersensitivity to capecitabine products or 5-FU, severe renal impairment ADVERSE EFFECTS: Anemia, Diarrhea, Hand-foot syndrome in sickle cell anemia, Hyperbilirubinemia, Myocardial infarction, Neutropenia, Thrombocytopenia 11 PRODUCT DESCRIPTION Captopril Carbamazepine Carbimazole TRADE NAME Capocard Capoten Carbatol Castor Oil Syrup 1mg/mL Tablet 25mg Tablet 50mg Tegretol Syrup 100mg/5mL Tablet 200mg Neo-Mercazole Tablet 5mg Carboplatin Carvedilol DOSAGE FORM/STRENGTH Inj. 10mg/mL Dilatrend Tablet 6.25mg Tablet 25mg Oil PRODUCT INFORMATION THERAPEUTIC USE: ACE Inhibitor, Antihypertensive, Cardiovascular Agent, Renal Protective Agent DOSE: Adult – Congestive heart failure, usually in combination with diuretics and digitalis, initial, 6.25 to 12.5mg orally 3 times a day, initial, 25 mg 3 times a day, maintenance, 50 to 100 mg 3 times a day, Max. 450 mg daily. Pedia – Safety and efficacy have not been established in pediatric patients CONTRA-INDICATIONS: history of angioedema, hypersensitivity to captopril/other ACE inhibitors ADVERSE EFFECTS: Agranulocytosis, Angioedema, Angioedema face, lips, throat (1 in 1000 patients; more frequent in Black patients), Intestinal angioedema, Neutropenia THERAPEUTIC USE: Anticonvulsant, Dibenzazepine Carboxamide, Neuropathic Pain Agent DOSE: Adult – Bipolar I disorder, acute manic and mixed episodes a)ORAL; (extended-release capsules) initial, 400 mg/day ORALLY in 2 divided doses, may increase dosage in increments of 200 mg/day up to a max of 1600 mg/day as needed; Epilepsy, Partial, generalized, and mixed types. ORAL; (suspension) initial, 1 teaspoon (100 mg) ORALLY 4 times a day for the first week, may increase dosage by 200 mg/day at weekly intervals (usual max dosage 1000 mg/day in children 12-15 years of age, 1200 mg/day in patients above 15 years of age, and up to 1600 mg/day in adults). Pedia – Epilepsy, Partial, generalized, and mixed types; ORAL; children up to 6 years of age (suspension), initial, 1020 mg/kg/day ORALLY in 4 divided doses, may increase dosage by 100 mg/day at weekly intervals as needed, do not exceed 35 mg/kg/day. CONTRA-INDICATIONS: bone marrow depression, history of previous, concomitant use of an MAOI, or use within 14 days of discontinuing an MAOI, concomitant use of nefazodone; decreased nefazodone plasma levels may reduce drug effectiveness, hypersensitivity to carbamazepine or tricyclic compounds ADVERSE EFFECTS: Acute intermittent porphyria, Acute renal failure, Agranulocytosis, Angioedema, Aplastic anemia, Atrioventricular block, Bone marrow depression, Cardiac dysrhythmia, Congestive heart failure, Drug-induced eosinophilia, Hepatitis, Hypocalcemia, Hyponatremia, Leukocytosis, Leukopenia, Nephrotoxicity, Pancytopenia, Stevens-Johnson syndrome, Syncope, Syndrome of inappropriate antidiuretic hormone secretion, Systemic lupus erythematosus, Aggravation, Thrombocytopenia, Toxic epidermal necrolysis THERAPEUTIC USE: Antithyroid Agent, Thionamide DOSE: Adult – Hyperthyroidism, Monotherapy, Usual starting doses of oral carbimazole for the treatment of hyperthyroidism are 20 to 60 milligrams daily, in 3 or 4 divided doses. Pedia - Oral doses of 0.75 to 1 milligram/kilogram/day in divided doses has been suggested for the initial treatment of hyperthyroidism in CHILDREN. NEONATES-Neonatal hyperthyroidism patients should receive 2.5 milligrams every 8 hours with a gradual reduction as symptoms are controlled CONTRA-INDICATIONS: Hypersensitivity to carbimazole or methimazole, Pregnancy; associated with congenital malformations but could be used during pregnancy, if medically necessary THERAPEUTIC USE: Antineoplastic Agent, Platinum Coordination Complex DOSE: Adult – Ovarian cancer, Advanced (as initial treatment in combination with other approved chemotherapy agents), 300 mg/m(2) on day 1 every 4 weeks for 6 cycles in combination with cyclophosphamide 600 mg/m(2) IV on day 1 every 4 weeks for 6 cycles. Pedia – safety and effectiveness in pediatric patients have not been established; 175-600 mg/m(2) IV has been used in various protocols CONTRA-INDICATIONS: hypersensitivity to cisplatin/platinum products or mannitol, severe myelosuppression/significant bleeding ADVERSE EFFECTS: Electrolyte imbalance, Immune hypersensitivity reaction, Myelosuppression, Peripheral neuropathy, Visual disturbance THERAPEUTIC USE: Alpha/Beta-Adrenergic Blocker, Antianginal, Antihypertensive, Cardiovascular Agent DOSE: Adult – Hypertension initial, 6.25 mg orally twice daily; dose may be doubled every 1-2 wks; max. dose, 25 mg orally twice daily. Pedia – Not FDA approved in children. Congestive heart failure, initial, 0.09 mg/kg orally twice daily; dose may be increased at 2-wk intervals as tolerated; maximum dose, 50 mg orally daily CONTRA-INDICATIONS: atrioventricular block, second- or third-degree, bronchial asthma or related bronchospastic condition; status asthmaticus resulting in death has been reported in 2 cases, cardiogenic shock, decompensated cardiac failure, with IV inotropic therapy, hepatic impairment, clinically manifest, hypersensitivity to carvedilol or any component of the product, sick sinus syndrome, sinus bradycardia, severe, patient without permanent pacemaker ADVERSE EFFECTS: Aplastic anemia, Asthma with status asthmaticus, Atrioventricular block, Erythema multiforme, Heart failure, Worsening, Stevens-Johnson syndrome, Toxic epidermal necrolysis THERAPEUTIC USE: Laxative, Stimulant DOSE: Adult – Constipation & Preparation of bowel for procedure, Preoperative- 15 to 60 mL ORALLY, dose varies depending on product. Pedia –a)(less than 2 yrs) 1 to 2mL ORALLY, up to 5mL, dose varies depending on product b)(2 to 12 yrs) 5 to 15mL ORALLY, dose varies depending on product c)(age 12 yrs and older) 15-60 mL ORALLY, dose varies depending on product CONTRA-INDICATIONS: abdominal pain, intestinal obstruction, nausea, vomiting, symptoms of appendicitis 12 PRODUCT DESCRIPTION Cefaclor TRADE NAME Biodroxil Ceclor Cloracef Tabiclor Ultracef DOSAGE FORM/STRENGTH Capsule 250mg Susp. 250mg/5mL Cefazolin Cefamizin Injection 500mg Injection 1gram Cefepime HCl Maxipime Injection 1gram Injection 2grams Cefotaxime Claforan Injection 1gram Ceftazidime Fortum Magnacef Negacef Injection 1gram Injection 2grams Ceftriaxone Enoxirt Rocephin Samixon Injection 1gram Cefuroxime Axetil Cefuzime Zinacef Zinnat Susp. 125mg/5mL Tablet 250mg Cefuroxime Sodium Cefuzime Zinacef Zinnat Injection 750mg Inj. 1.5grams PRODUCT INFORMATION THERAPEUTIC USE: 2nd Generation Cephalosporin, Antibiotic DOSE: Adult – Infection of skin &/or subcutaneous tissue, 375 mg extended-release ORALLY every 12 hr for 7 to 10 days; 250 to 500 mg regular-release ORALLY every 8 hr. Pedia – Infection of skin &/or subcutaneous tissue a)(1 month and older) 20 to 40 mg/kg/day regularrelease ORALLY divided every 8 hr, MAX 1 g/day b)(16 years and older) 375 mg extended-release ORALLY every 12 hr for 7 to 10 days. CONTRA-INDICATIONS: Hypersensitivity to cefaclor products/cephalosporins ADVERSE EFFECTS: Arthralgia, Arthritis, Erythema multiforme, Fever, More common in children, Rash, Serum sickness due to drug THERAPEUTIC USE: 1st Generation Cephalosporin, Antibiotic DOSE: Adult - Infection of skin &/or subcutaneous tissue a)(mild) 250 to 500 mg IV/IM every 8 h, depending on severity and type of infection b)(moderate to severe) 0.5 to 1 g IV/IM every 6 to 8 h, depending on severity and type of infection. Pedia – Infection of skin &/or subcutaneous tissue (1 month of age and older) 25 to 50 mg/kg/day IV/IM divided every 6 to 8 h, depending on severity of infection; dose may be increased to 100 mg/kg/day for severe infections. ADVERSE EFFECTS: Hepatotoxicity, Leukopenia, Pseudomembranous enterocolitis, Stevens-Johnson syndrome, Thrombocytopenia THERAPEUTIC USE: 4th Generation Cephalosporin, Antibiotic DOSE: Adult - Infection of skin &/or subcutaneous tissue (Moderate to Severe) 2 g IV every 12 hr for 10 days. Pedia - Infection of skin &/or subcutaneous tissue, (2 months of age and older, 40 kg or less), 50 mg/kg IV every 12 hr; maximum 2 g/dose ADVERSE EFFECTS: Encephalopathy, Myoclonus, Seizure, Renally impaired without dose adjustment THERAPEUTIC USE: 3rd Generation Cephalosporin, Antibiotic DOSE: Adult – Infection of skin &/or subcutaneous tissue a)(life-threatening) 2 g IV every 4 h b)(requiring higher doses) 2 g IV every 6 to 8 h; depending on type and severity of infection c)(moderate to severe) 1 to 2 g IV/IM every 8 h; depending on type and severity of infection d)(uncomplicated) 1 g IV/IM every 12 h; depending on type and severity of infection. Pedia - Infection of skin &/or subcutaneous tissue a)(neonates, premature and normal gestational age) 0 to 1 week of age, 50 mg/kg IV every 12 h; 1 to 4 weeks of age, 50 mg/kg IV every 8 h; depending on type and severity of infection b)(1 month to 12 years and under 50 kg) 50 to 180 mg/kg/day IV/IM divided into 4 or 6 equal doses depending on type and severity of infection; maximum 12 g/day; c)(life-threatening) over 12 years or 50 kg, 2 g IV every 4 h; maximum 12 g/day d)(requiring higher doses) over 12 years or 50 kg, 2 g IV every 6 to 8 h; depending on type and severity of infection e)(moderate to severe) over 12 years or 50 kg, 1 to 2 g IV/IM every 8 h; maximum 12 g/day; depending on type and severity of infection f)(uncomplicated) over 12 years or 50 kg, 1 g IV/IM every 12 h; maximum 12 g/day; depending on type and severity of infection ADVERESE EFFECTS: Agranulocytosis, Long-term therapy, Granulocytopenic disorder, Stevens-Johnson syndrome THERAPEUTIC USE: 3rd Generation Cephalosporin, Antibiotic DOSE: Adult – Infection of skin &/or subcutaneous tissue, mild, 0.5 g to 1 g IV/IM every 8 h severe life-threatening infections, 2 g IV every 8 h. Pedia – Infection of skin &/or subcutaneous tissue a)neonates (0 to 4 weeks), 30 mg/kg IV every 12 h b)1 month – 12 years, 30 to 50 mg/kg IV every 8 h; MAX 6 g/day ADVERSE EFFECTS: Asterixis, Disorder of neuromuscular transmission, Encephalopathy, Seizure THERAPEUTIC USE: 3rd Generation Cephalosporin, Antibiotic DOSE: Adult – Infection of skin &/or subcutaneous tissue,1 to 2 g IV/IM every 24 h or in divided doses twice a day; maximum 4 g/day. Pedia - Infection of skin &/or subcutaneous tissue,50 to 75 mg/kg/day IV/IM once daily or in divided doses every 12 h; maximum 2 g/day CONTRA-INDICATIONS: concurrent administration of calcium-containing solutions or products in newborns; risk of fatal salt precipitation in lungs and kidneys, hypersensitivity to cephalosporins, neonates, hyperbilirubinemic; increased risk of bilirubin encephalopathy (kernicterus), especially in premature neonates ADVERSE EFFECTS: Disorder of gallbladder, Reversible, Hemolysis, Immune-mediated, Kernicterus of newborn, Kidney finding, Lung finding THERAPEUTIC USE: 2nd Generation Cephalosporin, Antibiotic DOSE: Adult – Infection of skin &/or subcutaneous tissue, Uncomplicated, 250 mg or 500 mg orally twice a day for 10 days. Pedia - Infection of skin &/or subcutaneous tissue, Uncomplicated, 250 mg or 500 mg ORALLY twice a day for 10 days ADVERSE EFFECTS: Erythema multiforme, Hepatotoxicity, Hypersensitivity reaction, Stevens-Johnson syndrome, Toxic epidermal necrolysis THERAPEUTIC USE: 2nd Generation Cephalosporin, Antibiotic DOSE: Adult – Infection of skin &/or subcutaneous tissue 750 mg IV/IM every 8 hr. Pedia – Infection of skin &/or subcutaneous tissue 50 to 100 mg/kg/day in equally divided doses every 6 to 8 hours ADVERSE EFFECTS: Anaphylaxis, Erythema multiforme, Hypersensitivity reaction, Interstitial nephritis, Stevens-Johnson syndrome, Thrombocytopenia, Toxic epidermal necrolysis 13 PRODUCT DESCRIPTION Cephalexin Cetirizine HCl TRADE NAME Cefrin Cephadar Ceporex Keflex DOSAGE FORM/STRENGTH Capsule 250mg Capsule 500mg Susp. 250mg/5mL Finallerg Zyrtec Syrup 5mg/5mL Charcoal Activated Powder Chloral Hydrate Crystals Syrup 100mg/mL (50mL & 100mL) Chlorambucil Leukeran Tablet 2mg Chloramphenicol Chloroptic Riachol Spersanicol Eye Drops 0.5% Eye Oint. 1% Chloroquine Nivaquine Resochin Injection 200mg Syrup 50mg/5mL Tablet 250mg PRODUCT INFORMATION THERAPEUTIC USE: 1st Generation Cephalosporin, Antibiotic DOSE: Adult - Infection of skin &/or subcutaneous tissue, 250 mg ORALLY every 6 hr OR 500 mg ORALLY every 12 hr. Pedia – Infection of skin &/or subcutaneous tissue a)MILD TO MODERATE: 25 to 50 mg/kg/day ORALLY divided every 6 hr; maximum 4 g/day; depending on type and severity of infection b)SEVERE: 50 to 100 mg/kg/day ORALLY divided every 6 hr; depending on type and severity of infection c)total daily dose may be administered in two divided doses every 12 hr CONTRA-INDICATIONS: Hypersensitivity to cephalexin products/cephalosporins THERAPEUTIC USE: Antihistamine, Less-Sedating, Respiratory Agent DOSE: Adult – 5 -10 mg orally once daily. Pedia – Perennial allergic rhinitis a)(12 yrs and older) 5-10 mg orally once daily b)(6-11 yrs) 5-10 mg orally once daily (1-2 tsp (5 mg/5 mL solution) c)(2-5 yrs) 2.5-5 mg orally once daily (0.5-1 tsp (5 mg/5 mL solution) or 2.5 mg orally twice daily d)(6-23 months) 2.5 mg (0.5 tsp (5 mg/5 mL solution)) orally once daily; the dose in children 12 to 23 months of age can be increased to a max. dose of 5 mg per day given as 2.5 mg every 12 hr CONTRA-INDICATIONS: hypersensitivity to cetirizine or components, hypersensitivity to hydroxyzine THERAPEUTIC USE: Adsorbent, Gastrointestinal Agent, Nutriceutical DOSE: Adult - Diarrhea & Indigestion-520 mg ORALLY after meals or at the first sign of discomfort, MAX 4160 mg/day; Poisoning – Selective decontamination of the digestive tract, initial, ORAL, 30-100 g or 1-2 g/kg; repeat initial dose as soon as possible or 20-50 g every 26 hr. Pedia - Poisoning – Selective decontamination of the digestive tract a)to 1 yr of age, ORAL, as slurry in water 1 g/kg; may repeat half the initial dose every 2-6 hr as needed b)1-12 yr, ORAL, as slurry in water, 25-50 g or 1-2 g/kg; may repeat half the initial dose every 2-6 hr as needed c)13 yr and older, same as adult dosage d)continuous ORAL infusion, 0.25 to 0.5 g/kg/hr (up to 50 g/hr) CONTRA-INDICATIONS: absence of bowel sounds, gastrointestinal perforation, intestinal obstruction, recent surgery, risk of gastrointestinal hemorrhage ADVERSE EFFECTS: Electrolyte imbalance, GI obstruction, Hypotension THERAPEUTIC USE: Nonbarbiturate Hypnotic DOSE: Adult – Administration of analgesic; Adjunct – Preoperative care, 250 mg ORALLY 3 times a day after meals (max dose 2 g/day). Pedia – Administration of analgesic; Adjunct – Preoperative care 8 mg/kg body weight or 250 mg/m(2) of body surface area ORALLY 3 times a day (max dose 500 mg/dose) CONTRA-INDICATIONS: hypersensitivity to chloral hydrate, severe or marked hepatic or renal impairment ADVERSE EFFECTS: Confusion, Cutaneous hypersensitivity, Excitement, Unusual, Hallucinations THERAPEUTIC USE: Alkylating Agent, Antineoplastic Agent, Nitrogen Mustard DOSE: Adult - Chronic lymphoid leukemia a)0.1-0.2 mg/kg/day ORALLY for 3-6 wk b)continuous therapy, 0.03-0.1 mg/kg/day ORALLY c)intermittent, biweekly, or once-monthly pulse course, single dose of 0.4 mg/kg ORALLY, increase dose by 0.1 mg/kg to produce mild hematologic toxicity, until control of lymphocytosis or toxicity d)high dose therapy, 30 mg/m(2) ORALLY once every 2 wk has been used. Pedia – not FDA approved for use in children. Nephrotic syndrome – 100-200 mcg (0.1 to 0.2 mg) per kg of body weight per day ORALLY, in a single dose, for eight to twelve weeks ADVERSE EFFECTS: Drug fever, Erythema multiforme, Hallucinations, Hepatotoxicity, Immune hypersensitivity reaction, Infertility, Reversible or permanent, Leukemia, Myelosuppression, Peripheral neuropathy, Pneumonitis, acute, Secondary malignant neoplastic disease, Seizure, Skin reaction – finding, Stevens-Johnson syndrome, Toxic epidermal necrolysis THERAPEUTIC USE: Antibacterial, Antibiotic DOSE: Adult – Bacterial meningitis, 50 to 100 mg/kg/day IV divided every 6 h; depending on type and severity of infection. Pedia – Bacterial meningitis a)(neonates 14 days of age and younger) 25 mg/kg/day IV divided every 6 h b)(neonates over 14 days of age) up to 50 mg/kg IV divided every 6 h; depending on type and severity of infection c)(infants and children) 50 to 100 mg/kg/day IV divided every 6 h; depending on type and severity of infection ADVERSE EFFECTS: Aplastic anemia, Irreversible, Disorder of hematopoietic structure, Serious, some fatal, Gray syndrome from chloramphenicol administration in newborn, Newborns THERAPEUTIC USE: Amebicide, Extraintestinal, Aminoquinoline, Antimalarial, Antirheumatic DOSE: Adult - Malaria, acute-1000 mg chloroquine phosphate (600 mg as chloroquine base) ORALLY, then 500 mg chloroquine phosphate (300 mg as chloroquine base) ORALLY after 6 to 8 h, then 500 mg chloroquine phosphate (300 mg as chloroquine base) ORALLY once daily for 2 consecutive days; total dose, 2500 mg chloroquine phosphate (1500 mg as chloroquine base) in 3 days. Pedia - Malaria, acute-4 total doses: first dose, 10 mg/kg of chloroquine base (MAX, 600 mg base) ORALLY as a single dose followed by 5 mg/kg per dose ORALLY of chloroquine base (MAX, 300 mg base per dose) administered for 3 additional doses at 6 h, 24 h, and 36 h after the first dose CONTRA-INDICATIONS: hypersensitivity to 4-aminoquinoline compounds, retinal/visual field changes 14 PRODUCT DESCRIPTION TRADE NAME DOSAGE FORM/STRENGTH Chlorpheniramine Maleate Allerfin Inj. 10mg/mL Chlorpheniramine Maleate / Phenylephrine Sine-Up Syrup Chlorpromazine HCl Largactil Inj. 25mg/5mL Tablet 25mg Tablet 100mg Chlorzoxazone /Paracetamol Parafon Capsule Cholecalciferol Vi-De3 Drops 4500 iu Cholestyramine Questran Powder 4grams Cinnarizine Stugeron Tablet 25mg Ciprobay Eye Drops 0.3% Injection 200mg Tablet 250mg Tablet 500mg Ciprofloxacin Ciprogen Cipromax PRODUCT INFORMATION THERAPEUTIC USE: Alkylamine, Antihistamine, Cold/Cough Agent, Respiratory Agent DOSE: Adult - Allergic rhinitis-4 mg ORALLY every 4-6 hrs; maximum dose: 24 mg/day; sustained-release, 8 or 12 mg ORALLY every 8-12 hours; maximum dose: 24 mg/day; 5-40 mg IM, IV, SC as a single dose; maximum dose: 40 mg/day. Pedia –not recommended in children less than 6 yrs of age. Allergic rhinitis a)(6-11 yrs) 2 mg ORALLY every 4-6 hrs; maximum dose: 12 mg/day b)(12 yrs and over) sustainedrelease, 8 mg ORALLY every 12 hrs c)87.5 mcg/kg or 2.5 mg/m(2) SC 4 times daily CONTRA-INDICATIONS: hypersensitivity to chlorpheniramine THERAPEUTIC USE: For the symptomatic relief of upper respiratory tract disorders, includes allergic rhinitis, vasomotor rhinitis, common cold & influenza. DOSE: Adult – 10mL Three times daily. Pedia – 6-12 years old, 5mL 3 times daily; 2-5years old, 2.5mL 3 times daily; 6months-under 2years old, 1.25mL 3 times daily. CONTRA-INDICATIONS: Severe hypertension, monoamine oxidase inhibitors, who have previously exhibited intolerance to it or to any of its constituents. THERAPEUTIC USE: Aliphatic, Antiemetic, Antipsychotic, Gastrointestinal Agent, Phenothiazine DOSE: Adult - Nausea and vomiting a)ORAL; 10-25 mg ORALLY every 4-6 hr as needed b)RECTAL; 100 mg suppository every 6-8 hours as needed c)IM; 25 mg IM; if no hypotension occurs, give 25-50 mg IM every 3-4 hr as needed until vomiting stops, then switch to oral dosage d)IM; (during surgery) 12.5 mg IM repeated in 30 min if necessary and if no hypotension occurs e)IV; (during surgery) 2 mg IV per fractional injection (dilute to 1 mg/mL, i.e., 1 mL (25 mg) mixed with 24 mL of saline) at 2-minute intervals, do not exceed 25 mg. Pedia - Nausea and vomiting a)ORAL; 0.25 mg/pound of body weight b)RECTAL; 0.5 mg/pound of body weight every 6-8 hours as needed c)IM; 0.25 mg/pound of body weight IM every 6-8 hours as needed, max dose 40 mg/day for patients 6 months to 5 years (or 50 pounds) or 75 mg/day for patients 5-12 years (or 50-100 pounds) d)IM; (during surgery) 0.125 mg/pound body weight, repeat in 30 minutes if necessary and if no hypotension occurs e)IV (during surgery); 1 mg IV per fractional injection (dilute to 1 mg/mL, i.e., 1 mL (25 mg) mixed with 24 mL of saline) at 2-minute intervals, not to exceed recommended IM dosage CONTRA-INDICATIONS: hypersensitivity to chlorpromazine products, phenothiazines, myelosuppression, coma, severe CNS depression ADVERSE EFFECTS: Agranulocytosis, Cholestatic jaundice syndrome, Disorder of hematopoietic structure, Drug-induced lupus erythematosus, Systemic, Ineffective thermoregulation, Heatstroke or hypothermia, Leukopenia, Neuroleptic malignant syndrome, Obstipation, Paralytic ileus, Priapism, Prolonged QT interval, Seizure, Thrombocytopenia, Torsades de pointes THERAPEUTIC USE: Skeletal Muscle Relaxant, Centrally Acting DOSE: Adult - Musculoskeletal pain – 500 to 750 mg ORALLY 3 to 4 times daily CONTRA-INDICATIONS: hypersensitivity to chlorzoxazone products ADVERSE EFFECTS: Anaphylaxis, Gastrointestinal hemorrhage, Hepatotoxicity THERAPEUTIC USE: Nutritive Agent, Vitamin D (class) DOSE: Adult – Vitamin D deficiency; Prophylaxis-200 international units/day; ages 50-70, 400 international units/day; over age 70, 600 international units/day. Pedia – Vitamin D deficiency; Prophylaxis 200 international units/day CONTRA-INDICATIONS: Hypersensitivity to cholecalciferol, ergocalciferol, or vitamin D metabolites (eg, calcitriol, calcifediol, alfacalcidol, calcipotriol), Hypercalcemia (exacerbation with enhanced toxicity), Hypervitaminosis D (worsening of condition; pretherapy 25hydroxycholecalciferol levels should be considered in selected patients) THERAPEUTIC USE: Antihyperlipidemic, Bile Acid Sequestrant, Exchange Resin, Urinary Stone Agent DOSE: Adult - Hypercholesterolemia; Adjunct initial, 4 g ORALLY once or twice daily; maintenance, 8 to 16 g in divided doses, MAX 24 g daily; Pruritus of skin, Associated with partial biliary obstruction. Pedia – Hypercholesterolemia; Adjunct, 80 mg/kg anhydrous cholestyramine ORALLY 3 times a day CONTRA-INDICATIONS: complete biliary obstruction, hyperlipidemia types III, IV, or V, hypersensitivity to bile-sequestering resins THERAPEUTIC USE: Antihistamine, Calcium Channel Blocker DOSE: Adult – 75mg once or twice daily, followed by a maintenance dose of 75mg once daily. Pedia – 1mg/kg orally (maximum dose = 40 mg) relieved symptoms only during mild exposure to pollen. CONTRA-INDICATIONS: Prior history of hypersensitivity to cinnarizine or any of its components THERAPEUTIC USE: Antibiotic, Antitubercular, Fluoroquinolone DOSE: Adult – Anthrax, Postexposure; Prophylaxis, 400 mg IV every 12 hr for 60 days; 500 mg ORALLY every 12 hr for at least 60 days. Pedia - Cystic fibrosis, 30 mg/kg/day IV divided every 8 hr, MAX 1.2 g/day; 40 mg/kg/day ORALLY divided every 12 hr, MAX 2 g/day CONTRA-INDICATIONS: hypersensitivity to ciprofloxacin or other quinolones, concomitant tizanidine administration ADVERSE EFFECTS: Drug-induced psychosis, Immune hypersensitivity reaction, Serious, Peripheral neuropathy, Raised intracranial pressure, Seizure, Tendinitis, Traumatic or non-traumatic rupture of tendon 15 PRODUCT DESCRIPTION Cisatracurium Besylate TRADE NAME Nimbex DOSAGE FORM/STRENGTH Inj. 20mg/10mL Cisplatin Inj. 50mg/100mL Citric Acid Powder Citric Acid/Tartaric Acid / Na Bicarbonate Fawar Fruit Sachet 5grams Clarithromycin Klacid Susp. 125mg/5mL Tablet 250mg Clidinium Bromide/ Chlordiazepoxide Librax Tablet Clindamycin Dalacin - C Capsule 150mg Inj. 300mg/2mL Clobetasol Propionate Cloderm Dermovate Cream 0.05% PRODUCT INFORMATION THERAPEUTIC USE: Musculoskeletal Agent, Neuromuscular Blocker, Non-Depolarizing DOSE: Adult - dosage must be individualized, initial, 0.15 to 0.20 mg/kg IV bolus as components of a propofol/nitrous oxide/oxygen induction-intubation technique. Pedia - dosage must be individualized a)(age 1 to 23 mo) initial, 0.15 mg/kg IV over 5 to 10 s during either halothane or opioid anesthesia b)(age 2 to 12 y) initial, 0.1 to 0.15 mg/kg IV over 5 to 10 s during either halothane or opioid anesthesia c)(age 2 to 12 y) maintenance, initial IV infusion of 3 mcg/kg/min; then decrease to 1 to 2 mcg/kg/min IV infusion CONTRA-INDICATIONS: hypersensitivity to cisatracurium/bis-benzylisoquinolinium agents, hypersensitivity to benzyl alcohol (preservative in multi-dose vials) ADVERSE EFFECTS: Bradyarrhythmia, Bronchospasm THERAPEUTIC USE: Antineoplastic Agent, Platinum Coordination Complex DOSE: Adult – Gastric cancer docetaxel 75 mg/m(2) IV over 1 h followed by cisplatin 75 mg/m(2) IV over 1 to 3 h, both on day 1 only, followed by fluorouracil 750 mg/m(2)/day IV over 24 h daily for 5 d; repeat every 3 weeks. Pedia - safety and effectiveness not established in pediatric patients CONTRA-INDICATIONS: allergy to cisplatin/platinum-containing products, myelosuppression, pre-existing renal/hearing impairment ADVERSE EFFECTS: Cerebral herniation, Encephalopathy, Immune hypersensitivity reaction, Myelosuppression, Nausea, Nephrotoxicity, Ototoxicity, Reversible posterior leukoencephalopathy syndrome, Seizure, Vomiting THERAPEUTIC USE: Urinary Alkalinizer, Urinary Stone Agent and mild astringent THERAPEUTIC USE: Flatulence & indigestion associated with hyperchlorhydria. To render the urine alkaline in the treatment of inflammatory conditions of the urinary tract associated with acidic urine. In gout therapy, as an adjuvant with uricosuric agents. For the prevention of crystalluria during treatment with sulphonamides. DOSE: Adult – The contents of one sachet to be added to half a glass of water and taken while effervescing after meals. Pedia – (3 to 12years old) Half the adults dose THERAPEUTIC USE: Antibiotic, Macrolide DOSE: Adult - Acute exacerbation of chronic obstructive pulmonary disease - Bacterial infectious disease, 250-500 mg ORALLY twice daily for 7-14 days; extended-release tablets, 1000 mg ORALLY once daily for 7 days. Pedia - Infection of skin &/or subcutaneous tissue, Uncomplicated 15 mg/kg/DAILY (divided every 12 hours) for 10 days, MAX 1 g/day. CONTRA-INDICATIONS: concomitant cisapride, pimozide, astemizole, terfenadine, ergotamine, or dihydroergotamine; hypersensitivity to clarithromycin, erythromycin, or any macrolide antibiotics ADVERSE EFFECTS: Immune hypersensitivity reaction (Severe), Anaphylaxis, Stevens-Johnson syndrome, toxic epidermal necrolysis, Liver failure THERAPEUTIC USE: Antianxiety, Benzodiazepine, Long Acting DOSE: Adult – Anxiety mild to moderate anxiety; 5 or 10 mg ORALLY 3 to 4 times daily; severe anxiety; 20 or 25 mg ORALLY 3 to 4 times daily; geriatric patients or in the presence of debilitating disease: 5 mg ORALLY 2 to 4 times daily. Pedia - not recommended in pediatric patients under 6 yr of age, Anxiety about treatment, Preoperative, (6 y and older) 5 mg ORALLY 2 to 4 times daily; may be increased to 10 mg ORALLY 2 to 3 times daily ADVERSE EFFECTS: Agranulocytosis, Decreased liver function, Disorder of hematopoietic structure THERAPEUTIC USE: Antiacne, Antibacterial, Antibiotic , Antimalarial, Lincosamide DOSE: Adult - Infection of skin &/or subcutaneous tissue; serious infections: 150 to 300 mg ORALLY every 6 hours; depending on type of infection; more severe infections: 300 to 450 mg ORALLY every 6 hours; depending on type of infection. Pedia - Infection of skin &/or subcutaneous tissue serious infections: 8 to 16 mg/kg/day ORALLY divided every 6 to 8 hr; depending on type of infection; more severe infections: 16 to 20 mg/kg/day ORALLY divided every 6 to 8 hr; depending on type of infection CONTRA-INDICATIONS: Hypersensitivity to clindamycin or lincomycin, Clindamycin /benzoyl peroxide topical gel, clindamycin topical solution, gel and lotion, and clindamycin vaginal cream and suppositories: patients with a history of regional enteritis, ulcerative colitis, or antibiotic-associated colitis ADVERSE EFFECTS: Increased liver function test, Jaundice, Pseudomembranous enterocolitis THERAPEUTIC USE: Adrenal Glucocorticoid, Corticosteroid, Very Strong DOSE: Adult - Disorder of skin, Corticosteroid responsive (gel, cream, ointment, lotion, foam and scalp application), apply a thin layer TOPICALLY to affected area twice daily for a maximum of 2 wk, MAX 50 g/wk or 50 mL/wk. Pedia - TOPICAL, cream, gel, foam, ointment, scalp solution, safety and efficacy in children under 12 years of age have not been established ADVERSE EFFECTS: Secondary hypocortisolism 16 PRODUCT DESCRIPTION TRADE NAME DOSAGE FORM/STRENGTH Clomipramine HCl Anafranil Inj. 25mg/2mL Tablet 25mg Clonazepam Rivotril Drops 2.5mg/mL Tablet 0.5mg Tablet 2mg Clonidine Catapres Syrup 0.1mg/mL Tablet 150mcg Clopidogrel Plavix Tablet 75mg Canesten Cream 1% Ear Drops 10mg Vag.Tab. 500mg Clotrimazole Micoter Cloxacillin Prostaphlin – A Capsule 250mg Injection 250mg Susp. 125mg/5mL Co-Trimoxazole Bactrim Bactrim DS Septrin Septrin DS Trimol Inj. 480mg/5mL Susp. 240mg/5mL Tablet 480mg Tablet 960mg Colchicine Tablet 500mcg PRODUCT INFORMATION THERAPEUTIC USE: Antidepressant, Tricyclic, Central Nervous System Agent DOSE: Adult – Depression initial, 75 mg/day ORALLY (3 divided doses); may increase dosage slowly as needed and tolerated to a range of 100-250 mg/day (3 divided doses); Pedia - safety and effectiveness in children up to 10 years of age have not been established. Depression, 20-30 mg/day ORALLY; may increase dosage by 10 mg/day at 4-5 day intervals as needed and tolerated CONTRA-INDICATIONS: coadministration with an MAOI or use within 14 days of discontinuing an MAOI; may cause serious reactions, hypersensitivity to clomipramine hydrochloride or other tricyclic antidepressant, myocardial infarction, during the acute recovery period ADVERSE EFFECTS: Agranulocytosis, Depression, worsening, Hepatotoxicity, Leukopenia, Myocardial infarction, Orthostatic hypotension, Pancytopenia, Seizure, Suicidal thoughts, Suicide, Thrombocytopenia THERAPEUTIC USE: Antianxiety, Anticonvulsant, Benzodiazepine, Short or Intermediate Acting DOSE: Adult – Seizure initial, 0.5 mg orally 3 times a day, maintenance, may increase daily dose by 0.5 to 1 mg orally every 3 days to a MAX total daily dose of 20 mg (divided into 3 daily doses). Pedia – Seizure up to 10 y of age or up to 30 kg, initial, 0.01 to 0.03 mg/kg/day orally divided into 2 to 3 daily doses; up to 10 y of age or up to 30 kg, maintenance, may increase daily dose by 0.25 to 0.5 mg orally every 3 days to MAX total daily dose of 0.1 to 0.2 mg/kg/day (divided into 3 daily doses) CONTRA-INDICATIONS: acute narrow angle glaucoma, hypersensitivity to clonazepam or benzodiazepines, severe liver disease THERAPEUTIC USE: Alpha-2 Adrenergic Agonist, Analgesic, Anesthetic Adjunct Antihypertensive, Antimigraine, Cardiovascular Agent, Diagnostic Agent, Pheochromocytoma DOSE: Adult - Essential hypertension, initial, 0.1 mg orally twice daily (morning and bedtime); increase in increments of 0.1 mg daily orally at weekly intervals, usual daily dose is 0.2-0.6 mg in divided doses; MAX 2.4 mg/day. Pedia - Attention deficit hyperactivity disorder initial, 50mcg orally daily, increased in 50mcg increments; maintenance, 5 mcg/kg/day orally in 4 divided doses (200-600 mcg/day) ADVERSE EFFECT: Atrioventricular block THERAPEUTIC USE: ADP-Induced Aggregation Inhibitor, Platelet Aggregation Inhibitor DOSE: Adult - Acute coronary syndrome, Non-ST segment elevation - Thrombotic disorder; Prophylaxis initial, 300 mg ORALLY once; with aspirin 75 mg to 325 mg ORALLY; maintenance, 75 mg ORALLY once daily with aspirin 75 mg to 325 mg ORALLY once daily. Pedia - safety and efficacy in pediatric patients not established CONTRA-INDICATIONS: bleeding, active (such as peptic ulcer or intracranial hemorrhage) hypersensitivity to clopidogrel or any component of the product ADVERSE EFFECTS: Abnormal renal function, Acute renal failure, Agranulocytosis, Anaphylaxis, Atrial fibrillation, Congestive heart failure, Epidural hematoma, Erythema multiforme, Gastrointestinal hemorrhage, Gastrointestinal ulcer, Hepatitis, Intracranial hemorrhage, Intraocular hemorrhage, Liver function tests abnormal, Non-cardiogenic pulmonary edema, Thrombotic thrombocytopenic purpura THERAPEUTIC USE: Antifungal, Imidazole DOSE: Adult - Candidal vulvovaginitis, insert 1 500 mg tablet INTRAVAGINALLY at bedtime for 1 dose; TOPICAL, apply thin layer of 1% cream twice daily for up to 4 wks; Ear Drops – Instill 1 to 2 drops in the ear 3 or 4 times daily, continue for 2weeks after signs of infection disappears. Pedia- Candidal vulvovaginitis,12 yr and older, insert 1 applicatorful of 2% cream INTRAVAGINALLY at bedtime for 3 days; Candidiasis, topical apply thin layer of 1% cream twice daily for up to 4 wks THERAPEUTIC USE: Antibiotic, Penicillin, Penicillinase-Resistant DOSE: Adult - 250 to 500 mg IV/ORALLY every 6 hours; maximum 6 g/day; depending on type and severity of infection. Pedia - over 20 kg: 250 to 500 mg IV/ORALLY every 6 hours; maximum 6 g/day; depending on type and severity of infection; less than 20 kg: 25 to 50 mg/kg/day IV/ORALLY (in equally divided doses every 6 hours); dose may be increased in severe infections CONTRA-INDICATIONS: hypersensitivity to cloxacillin or other penicillins ADVERSE EFFECTS: Agranulocytosis, Hepatotoxicity, Immune hypersensitivity reaction, Myelosuppression, Neutropenia, Pseudomembranous enterocolitis THERAPEUTIC USE: Antibiotic, Sulfonamide Combination DOSE: Adult - 1 double-strength tablet or 2 single-strength tablets or 20 mL suspension ORALLY every 12 hours for 10 to 14 days. Pedia (2 months of age and older) 8 mg/kg TMP component/day ORALLY divided every 12 hours for 10 days. CONTRA-INDICATIONS: hypersensitivity to sulfonamides or trimethoprim, infants less than 2 months; PCP prophylaxis can begin at 1 month of age, pregnant patients at term, nursing mothers, megaloblastic anemia due to folate deficiency ADVERSE EFFECTS: Agranulocytosis, Aplastic anemia, Disorder of hematopoietic structure, Hepatic necrosis, Fulminant, Immune hypersensitivity reaction (Severe), Stevens-Johnson syndrome, toxic epidermal necrolysis THERAPEUTIC USE: Antigout DOSE: Adult - Gout; Treatment and Prophylaxis a)(Treatment)ORAL, initial, 0.5-1.2 mg, then 0.5-0.6 mg every hr or 1-1.2 mg every 2 hr until symptoms abate or diarrhea occurs (usual dose 4-8 mg); MAX 8 mg ORALLY per attack; a 3-day colchicine-free interval should follow each ORAL course b)(Treatment) IV, initial, 2 mg over 2-5 min, then 0.5 mg every 6 hr or 1 mg every 6-12 hr until satisfactory response; MAX IV 4 17 PRODUCT DESCRIPTION TRADE NAME DOSAGE FORM/STRENGTH Colomycin Injection 500,000iu Conjugated Oestrogens Premarin Tablet 0.625mg Tablet 1.25mg Vaginal Cream 0.625mg/g Crotamiton Eurax Cream 10% Lotion 10% Cyclopentolate HCl Cyclogyl Eye Minims 0.5% Eye Minims 1% Endoxan Injection 200mg Injection 500mg Injection 1gram Tablet 50mg Sandimmun Capsule 25mg Capsule 50mg Capsule 100mg Inj. 50mg/mL Colistimethate Cyclophosphamide Cyclosporin PRODUCT INFORMATION mg/24 hr or per attack; a 7-day colchicine-free interval should follow each full IV course (4 mg) c)(prophylaxis) ORAL, less than 1 acute attack/yr, 0.5-0.6 mg/day 3-4 times/wk; more than 1 acute attack/yr, 0.5-0.6 mg/daily up to 1.5-1.8 mg/day d)(prophylaxis) IV, 0.5-1 mg once or twice daily e)(preoperative prophylaxis) ORAL, 0.5-0.6 mg 3 times daily, 3 days before through 3 days after surgery. Pedia - not FDA approved in children. Familial Mediterranean fever; initial, 0.5 mg/day ORALLY; maintenance, (5 yr or younger) 0.5 mg/day ORALLY; maintenance, (5 yr or older) 0.5 mg twice a day ORALLY; MAX 2 mg/day CONTRA-INDICATIONS: blood dyscrasias, cardiac disease, severe, gastrointestinal disease, severe, hepatic failure, hypersensitivity to colchicine, renal disease, severe ADVERSE EFFECTS: Myelosuppression, Neuromyopathy THERAPEUTIC USE: Antibiotic DOSE: Adult & Pedia - Disease due to Gram-negative bacteria, Pseudomonas aeruginosa, Enterobacter aerogenes, Escherichia coli, and Klebsiella pneumoniae, 2.5 to 5 mg/kg/day IM or IV in 2 to 4 divided doses, depending on severity of infection; MAX 5 mg/kg/day CONTRA-INDICATIONS: history of sensitivity to colistimethate or its components ADVERSE EFFECTS: Acute respiratory failure, Nephrotoxicity, Neurotoxicity, Respiratory tract paralysis THERAPEUTIC USE: Contraceptive, Endocrine-Metabolic Agent, Estrogen, Female Reproductive Agent, Musculoskeletal Agent DOSE: Adult - Postmenopausal osteoporosis; Prophylaxis, initial, 0.3 mg ORALLY daily given continuously or in cyclical regimens (25 days on, 5 days off); adjust dose based upon the individual clinical and bone mineral density responses of the patient; adjust to lowest level that will provide effective control. Pedia - safety and efficacy in children not established; however, estrogen therapy has been used for the induction of puberty in adolescents with some forms of pubertal delays CONTRA-INDICATIONS: arterial thromboembolic disease, breast cancer, known, suspected or history of; except in appropriately selected patients being treated for metastatic disease,deep vein thrombosis/pulmonary embolism, genital bleeding, undiagnosed abnormal, hypersensitivity to conjugated estrogens or product ingredients, liver dysfunction or disease, pregnancy ADVERSE EFFECTS: Anaphylaxis, Body fluid retention, Breast cancer, Breast cancer, Cerebrovascular accident, Cervical cancer, Cervical cancer, Dementia, Diabetes mellitus, Disorder of gallbladder, Heart disease, Hypercalcemia, Hypertension, Impaired cognition, Malignant neoplasm of endometrium of corpus uteri, Myocardial infarction, Ovarian cancer, Pancreatitis, Pulmonary embolism, Thrombosis of retinal vein, Venous thromboembolism THERAPEUTIC USE: Scabicide DOSE: Adult- Pruritus of skin completely rub lotion into affected areas, repeat as necessary; Scabies apply cream or lotion topically to entire body from the chin down (do not rinse off), 24 h later apply cream or lotion a second time and leave on the skin for 48 h; a cleansing bath should be taken 48 h after the last application. Pedia - not FDA approved in pediatric patients CONTRA-INDICATIONS: hypersensitivity to crotamiton THERAPEUTIC USE: Antimuscarinic, Mydriatic-Cycloplegic DOSE: Adult - Cycloplegic refraction, 1 to 2 drops of 0.5% to 2% solution in eye(s); may repeat after 5 to 10 min if needed. Pedia Cycloplegic refraction a)(children) 1 to 2 drops of 0.5% to 2% solution in eye(s); may repeat with 0.5% or 1% solution after 5 to 10 min if needed b)(small infants) single instillation of 1 drop of 0.5% solution in the eye; apply pressure to nasolacrimal sac for 2 to 3 min; observe infant closely for at least 30 min for signs or symptoms of systemic absorption CONTRA-INDICATIONS: narrow-angle glaucoma or anatomical narrow angles, hypersensitivity to cyclopentolate products ADVERSE EFFECTS: Ataxia, Confusion, Conjunctivitis, Psychotic disorder, Raised intraocular pressure, Seizure, Tachyarrhythmia, Vasodilatation THERAPEUTIC USE: Alkylating Agent, Antineoplastic Agent, Antirheumatic, Cytotoxic, Nitrogen Mustard DOSE: Adult & Pedia – (single agent) 40 to 50 mg/kg IV in divided doses over 2 to 5 days or 10 to 15 mg/kg IV every 7 to 10 days OR 3 to 5 mg/kg IV twice weekly; (single agent) oral cyclophosphamide is usually administered at dosages in the range of 1 to 5 mg/kg/day for both initial and maintenance dosing. CONTRA-INDICATIONS: hypersensitivity to cyclophosphamide products, severely depressed bone marrow function ADVERSE EFFECTS: Azoospermia, Cardiomyopathy, Hemorrhagic cystitis, Infectious disease, Interstitial pneumonia, Oligozoospermia, Stevens-Johnson syndrome, Toxic epidermal necrolysis THERAPEUTIC USE: Anti-Inflammatory, Immune Suppressant DOSE: Adult - Cardiac transplant rejection; Treatment and Prophylaxis, initial, (preoperative): 15 mg/kg ORALLY 4-12 hr before transplant; continue same dose as daily dose for 1-2 wk; initial, (preoperative): 5-6 mg/kg/day IV 4-12 hr before transplant; continue same initial dose as daily dose until patient can tolerate oral formulation; maintenance, decrease by 5% per week to 5-10 mg/kg/day ORALLY; doses are then adjusted to attain center-defined trough blood concentrations. Pedia - 6 mo and older, initial, (preoperative): 15 mg/kg ORALLY 4-12 hr before transplant; continue dose for 1-2 wk; 6 mo and older, initial, (preoperative): 5-6 mg/kg/day IV 4-12 hr before transplant; continue dose until patient can tolerate oral formulation. 18 PRODUCT DESCRIPTION TRADE NAME DOSAGE FORM/STRENGTH Cytarabine Cytosar Injection 100mg Injection 500mg Injection 1gram D-Penicillamine Artamine Capsule 250mg Dacarbazine Deticene Injection 100mg Injection 200mg Dantrolene Sodium Dantrium Injection 20mg Daunorubicin Daunoblastina Injection 20mg Desferrioxamine Mesylate Desferal Injection 500mg PRODUCT INFORMATION ADVERSE EFFECTS: Anaphylaxis, With IV use, Gingival enlargement, Hemolytic uremic syndrome, Hepatotoxicity, Hyperkalemia, Hypertension, Hypomagnesemia, Infectious disease, Nephrotoxicity, Pancreatitis, Paresthesia, Post-transplant lymphoproliferative disorder THERAPEUTIC USE: Antimetabolite, Antineoplastic Agent DOSE: Adult & Pedia - Acute myeloid leukemia, In combination with other approved chemotherapy agents for remission induction a)100 mg/m(2)/day continuous IV infusion for 7 days or 100 mg/m(2) IV every 12 hr for 7 days b)high dose induction therapy, 3 g/m(2) IV infused over 1-3 hr every 12 hr for 2-6 days ADVERSE EFFECTS: Anaphylaxis, Anemia, Bleeding, Infectious disease, Kidney disease, Leukopenia, Neuropathy, Sepsis, Thrombocytopenia THERAPEUTIC USE: Antirheumatic, Heavy Metal Chelator-Antagonist, Renal-Urologic Agent DOSE: Adult - Rheumatoid arthritis (Severe), Active disease that has failed to respond to conventional therapy a)initial, 125 to 250 mg/day ORALLY; increase dose at 1 to 3 month intervals by 125 or 250 mg/day, as patient response and tolerance indicate; if no improvement and no serious toxicity after 2 to 3 months, increases of 250 mg/day at 2 to 3 month intervals may be continued until remission or toxicity; b)maintenance, 500 to 1500 mg/day; typical range 500 to 750 mg/day. Pedia - Copper measurement, urine 500 mg ORALLY immediately before the second (post-baseline) 24-h urine sample collection and 12 h later, during the second (post-baseline) 24-h urine sample collection CONTRA-INDICATIONS: breastfeeding, during pregnancy, except for treatment of Wilson's disease and certain cases of cystinuria, hypersensitivity to penicillamine products, penicillamine-related aplastic anemia/agranulocytosis, rheumatoid arthritis patients with history or evidence of renal insufficiency ADVERSE EFFECTS: Agranulocytosis, Aplastic anemia, Goodpasture's syndrome, Myasthenia gravis, Obliterative bronchiolitis, Optic neuritis, Oral lichenoid reaction, Pemphigus, Peripheral motor neuropathy, Proteinuria, Renal glomerular disease, Renal vasculitis, Sensory neuropathy, Thrombocytopenia, Tinnitus THERAPEUTIC USE: Alkylating Agent, Antineoplastic Agent, Imidazole Carboxamide DOSE: Adult - Hodgkin's disease, Second line, in combination with other effective agents a)150 mg/m(2)/day for 5 days in combination with other effective drugs; may repeat every 4 wks b) 375 mg/m(2) on day 1 in combination with other effective drugs; repeat every 15 days. Pedia - not FDA approved for children CONTRA-INDICATIONS: hypersensitivity to dacarbazine products ADVERSE EFFECTS: Anaphylaxis, Cerebral hemorrhage, Hepatic necrosis, Hepatic vein thrombosis, Hepatotoxicity, Myelosuppression, Photosensitivity, Seizure THERAPEUTIC USE: Skeletal Muscle Relaxant, Direct Acting DOSE: Adult & Pedia - Malignant hyperthermia, 1 mg/kg IV by continuous rapid intravenous push and continue until symptoms subside or to a max cumulative dose of 10 mg/kg; discontinue all anesthetic agents and administer 100% oxygen (recommended). CONTRA-INDICATIONS: active hepatic disease (acute hepatitis, active cirrhosis), no contraindications for the use of dantrolene sodium IV in the treatment of malignant hyperthermia crisis, not for use where spasticity is utilized to sustain upright balance/posture in ambulation or when spasticity is utilized to obtain or maintain increased function ADVERSE EFFECTS: Abnormal blood pressure, Aplastic anemia, Disease of liver, Fatal and non-fatal, Heart failure, Leukopenia, Malignant lymphoma - small lymphocytic, Phlebitis, Tachyarrhythmia, Thrombocytopenia THERAPEUTIC USE: Anthracycline, Antineoplastic Agent DOSE: Adult - Acute lymphoid leukemia, For remission induction in combination with other chemotherapy agents, 45 mg/m(2)/day IV on days 1, 2, and 3 and vincristine 2 mg IV on days 1, 8, and 15. On days 1 through 22, prednisone 40 mg/m(2)/day ORALLY is administered, then tapered between days 22 to 29; L-asparaginase 500 International Units/kg/day IV on days 22 to 32. Pedia - Acute lymphoid leukemia, For remission induction in combination with other chemotherapy agents, 25 mg/m(2) IV and vincristine 1.5 mg/m(2) IV on day 1 of every week along with daily administration of prednisone 40 mg/m(2) ORALLY; four to six 7-day courses are generally required to produce a complete remission ADVERSE EFFECTS: Cardiotoxicity, Hyperuricemia, Myelosuppression THERAPEUTIC USE: Heavy Metal Chelator-Antagonist DOSE: Adult - Iron toxicity, chronic - Thalassemia major a)0.5 to 1 g IM daily, plus 2 g IV per unit of transfused blood; MAX rate, 15 mg/kg/h; MAX dose, 1 g/day with no transfusion, 6 g/day if 3 or more units of transfused blood or packed red blood cells b)1 to 2 g (20 to 40 mg/kg/day) subQ over 8 to 24 h. Pedia - Iron toxicity, chronic - Thalassemia major (age 3 years and older) 20 to 40 mg/kg per day SC over 8 to 24h CONTRA-INDICATIONS: anuria, severe renal disease ADVERSE EFFECTS: Deferoxamine Mesylate, Cardiac complication, Eye / vision finding, Hypotension, Immune hypersensitivity reaction, Mucormycosis, Ototoxicity, Shock, Tachyarrhythmia 19 PRODUCT DESCRIPTION TRADE NAME Desflurane Desmopressin DOSAGE FORM/STRENGTH Inhalation Minirin Decadron Inj. 4mcg/mL Nasal Drops 100mcg Nasal Spray 0.1mg Spersadex Eye Drops 1mg/mL Inj. 8mg/2mL Tablet 0.5mg Tablet 1.5mg Dexmedetomidine HCl Precedex Inj. 100mcg/mL Dexpanthenol Bepanthen Dexipan Panthenol Cream 5% Dexamethasone Dextromethorphan HBr Oradoxon Kafosed Romin Syrup 15mg/5mL PRODUCT INFORMATION THERAPEUTIC USE: General Anesthesia, Haloalkane, Volatile Liquid DOSE: Adult - General anesthesia a)induction, usually delivered at an initial inspired concentration of 3% in oxygen or nitrous oxide/oxygen and increased by 0.5%-1% every 2-3 breaths or as tolerated (up to 11%), until loss of consciousness b)maintenance, inhaled in concentrations of 2.5%-8.5% with or without concomitant nitrous oxide. Pedia - General anesthesia, maintenance, inhaled in concentrations of 5.2%-10% with or without concomitant nitrous oxide CONTRA-INDICATIONS: hypersensitivity to desflurane or other halogenated agent, malignant hyperthermia, known or suspected genetic susceptibility ADVERSE EFFECTS: Apnea, Bradyarrhythmia, Cardiac dysrhythmia, Complication of anesthesia, During induction, Desaturation of blood, Hepatic necrosis, Hepatitis, Hyperkalemia, Perioperative, Hypertension, Interrupted breathing, Laryngeal spasm, Liver failure, Malignant hyperthermia, Nephrotoxicity, Pharyngitis, Sinus arrhythmia, Tachycardia THERAPEUTIC USE: Endocrine-Metabolic Agent, Hemostatic, Vasopressin (class) DOSE: Adult - Hemophilia A, With factor VIII levels greater than 5%, 0.3 mcg/kg diluted in 50 mL sterile physiological saline, infused IV slowly over 15 to 30 minutes; monitor patient to determine necessity of further doses, tachyphylaxis may occur if given more often than every 48 hours; Primary nocturnal enuresis -initial, 20 mcg (0.2 mL) INTRANASALLY at bedtime; maintenance 10 to 40 mcg INTRANASALLY at bedtime for 4 to 8 weeks. Pedia - von Willebrand disease type 1 (Mild to Moderate), With factor VIII levels greater than 5%, 3 months and older: 0.3 mcg/kg diluted in sterile physiological saline, infused IV slowly over 15 to 30 minutes; dilute in 50 mL for children over 10 kg and in 10 mL for children 10 kg or less; monitor patient to determine necessity of further doses, tachyphylaxis may occur if given more often than every 48 hours; Primary nocturnal enuresis - 6 years of age and older: initial, 20 mcg (0.2 mL) INTRANASALLY at bedtime; maintenance 10 to 40 mcg INTRANASALLY at bedtime for 4 to 8 weeks CONTRA-INDICATIONS: hypersensitivity to desmopressin or any components of desmopressin products, patients with moderate to severe renal impairment ADVERSE EFFECTS: Abnormal blood pressure, Anaphylaxis, Hyponatremia, Palpitations, Seizure, Tachyarrhythmia, Thrombotic disorder, Water intoxication syndrome THERAPEUTIC USE: Adrenal Glucocorticoid, Antiemetic, Anti-Inflammatory, Corticosteroid, Weak, Diagnostic Agent, Adrenocortical Function, Endocrine-Metabolic Agent, Immune Suppressant, Ophthalmologic Agent, Otic Agent DOSE: Adult - Allergic disorder a)ORAL; 0.75 to 9 mg/day, vary dose according to patient response b)IV or IM; 0.5 to 9 mg/day; vary dose depending on patient response c)IM followed by ORAL; first day, dexamethasone sodium phosphate 4-8 mg IM; second and third days, 4 tablets (0.75 mg each) in 2 divided doses each day; fourth day, 2 tablets in 2 divided doses; fifth and sixth days, 1 tablet each day; seventh day, no treatment. Pedia - Allergic disorder a)ORAL; 0.02 to 0.3 mg/kg/day in 3 or 4 divided doses, vary dose according to patient response b)IV or IM; 0.5 to 9 mg/day; vary dose depending on patient response CONTRA-INDICATIONS: acute infectious stages of vaccinia, varicella, and many other viral, diseases of the cornea and conjunctiva, epithelial herpes simplex keratitis (dendritic keratitis), fungal diseases of ocular or auricular structures, hypersensitivity to dexamethasone, mycobacterial infection of the eye, perforation of a drum membrane, systemic fungal infections, Serious Adverse Effects, Glaucoma, HyperglycemiaPrimary adrenocortical insufficiency THERAPEUTIC USE: Alpha-2 Adrenergic Agonist, Analgesic, Anesthetic Adjunct Sedative DOSE: Adult - Sedation, Intubated/mechanically ventilated ICU patients, initial, 1 mcg/kg IV over 10 min; followed by 0.2 to 0.7 mcg/kg/hr continuous IV infusion for a max of 24 hr. Pedia - safety and effectiveness in children less than 18 years of age have not been established CONTRA-INDICATIONS: hypersensitivity to dexmedetomidine ADVERSE EFFECTS: Acidosis, Anemia, Apnea, Atrial fibrillation, Atrioventricular block, Bradyarrhythmia, Bronchospasm, Cardiac arrest, Dyspnea, Hypercapnia, Hyperkalemia, Hypertension, Aggravated, Hypotension, Hypoventilation, Hypoxia, Infectious disease, Leukocytosis, Oliguria, Pleural effusion, Pulmonary congestion, Pulmonary edema, Respiratory acidosis, Supraventricular tachycardia,Ventricular arrhythmia, Ventricular tachycardia THERAPEUTIC USE: Emollient, Stimulant, Gastrointestinal DOSE: Adult - Topical application route, Dexpanthenol is used topically in 2% to 5% creams, lotions, solutions or ointments for the treatment of minor skin disorders such as itching, mild eczema, stings, bites, poison ivy, poison oak, and diaper rash and to improve wound healing CONTRA-INDICATIONS: Hemophilia, Ileus due to mechanical obstruction, Paraben sensitivity (dexpanthenol inj. may contain parabens) THERAPEUTIC USE: Antitussive DOSE: Adult – Cough 10-20 mg ORALLY every 4hrs or 30 mg ORALLY every 6-8hrs; (sustained-release) 60 mg ORALLY twice daily, maximum dose, 120 mg/day. Pedia – Cough a)(6-12yrs) 5-10 mg ORALLY every 4hrs or 15 mg ORALLY every 6-8hrs; maximum dose: 60 mg/day b)(2-6yrs) 2.5-5 mg ORALLY every 4hrs or 7.5 mg ORALLY every 6-8hrs; maximum dose: 30 mg/day c)(sustained-release) 6-12yrs: 30 mg ORALLY twice daily d)(sustained-release) 2-6yrs: 15 mg ORALLY twice daily CONTRA-INDICATIONS: hypersensitivity to dextromethorphan, Co-administration with monoamine oxidase inhibitors 20 PRODUCT DESCRIPTION Dextromethorphan/ Amm. Chloride/Citric Acid TRADE NAME Romilar Dextrose Diazepam Digoxin Syrup 15mg/5mL Injection 50% Stesolid Valium Diazoxide Diclofenac Sodium DOSAGE FORM/STRENGTH Inj. 10mg/2mL Syrup 1mg/mL (50mL & 100mL) Tablet 5mg Tablet 50mg Clofen Diclogesic Diclogesic Retard Ocugesic, Olfen Rofenac Voltaren Voltaren EmuGel Votrex Eye Drops 0.1% Gel 1% Inj. 75mg/3mL Supp. 12.5mg Supp. 50mg Supp. 100mg Tablet 25mg Tablet 50mg Tablet 100mg Lanoxin Elixir 0.05mg/mL Inj. 0.5mg/2mL Tablet 0.0625mg Tablet 0.25mg PRODUCT INFORMATION THERAPEUTIC USE: For relief of lesion related or nonproductive cough associated with tracheitis or bronchitis; for habit cough as a sequel of whooping cough or in measles. DOSE: Adult – 1 to 2 teaspoonfuls two to four times daily. Pedia – over 2years old, 1/2 teaspoonful two to four times daily (according to age). May be taken undiluted or in water, tea, milk, fruit juice, etc., and should preferably be taken after meals. CONTRA-INDICATIONS: Hypersensitivity to one or more ingredients, severely impaired hepatic or renal function, monoamine oxidase inhibitors. ADVERSE EFFECTS: Mild & transient tiredness, nausea & dizziness may occur after intake, urticaria, skin skin rashes. THERAPEUTIC USE: Diagnostic Agent, Nutritive Agent, Osmotherapy Agent, Parenteral Solution DOSE: Adult - Glucose tolerance test a)Diabetes mellitus (diagnosis): 75 g anhydrous glucose (dextrose) dissolved in water ORALLY, as one-time dose for oral glucose tolerance test b)Gestational diabetes mellitus (diagnosis), one-step approach: 100 g anhydrous glucose (dextrose) dissolved in water ORALLY, as one-time dose for oral glucose tolerance test c)Gestational diabetes mellitus (diagnosis), two-step approach: 50 g anhydrous glucose (dextrose) dissolved in water ORALLY as one-time dose for glucose challenge test, then perform the 100g oral glucose tolerance test on those who exceeded the threshold value on the glucose challenge test CONTRA-INDICATIONS: a)Do not use concentrated solutions of dextrose in patients with: anuria, diabetic coma and hyperglycemia, intracranial or intraspinal hemorrhage, delirium tremens in dehydrated patients, glucose-galactose malabsorption syndrome b)Allergy to corn or corn products THERAPEUTIC USE: Antianxiety, Anticonvulsant, Benzodiazepine, Long Acting, Skeletal Muscle Relaxant DOSE: Adult – Anxiety a)2 to 10 mg ORALLY 2 to 4 times a day depending on symptom severity b)2 to 10 mg IM or IV every 3 to 4 h if needed depending on symptom severity . Pedia – Anxiety,initial, 1 to 2.5 mg ORALLY 3 to 4 times daily; increase gradually as needed CONTRA-INDICATIONS: acute narrow angle glaucoma, hypersensitivity to diazepam products, patients less than 6 months of age, untreated open angle glaucoma THERAPEUTIC USE: Antihypertensive, Gastrointestinal Agent, Glucose Regulation, Antihypoglycemic, Thiazide Related DOSE: Adult & Pedia - Malignant hypertension 1-3 mg/kg (MAX 150 mg) IV bolus every 5-15 min; MAX 1.2 grams per day. CONTRA-INDICATIONS: functional hypoglycemia, hypersensitivity to diazoxide, other thiazides, or sulfonamides, hypertension associated with aortic coarctation or arteriovenous shunt ADVERSE EFFECTS: Body fluid retention, Bowel obstruction, Cardiac arrest, Congestive heart failure, Diabetic ketoacidosis, Extrapyramidal disease, Hypernatremia, Neutropenia, Pancreatitis, Thrombocytopenia THERAPEUTIC USE: Analgesic, Antirheumatic, Central Nervous System Agent Musculoskeletal Agent, NSAID, Ophthalmologic Agent DOSE: Adult - Rheumatoid arthritis, Delayed-release, 100-200 mg/day orally in 3 or 4 divided doses (Max. 225mg/day); Extended- release, 75 mg orally twice a day or 100 mg orally once or twice a day; Enteric-coated 150-200 mg/day in divided doses; Light intolerance - Pain in eye - Refractive keratoplasty, instill 1-2 drops into affected eye before surgery and four times a day beginning within 15 minutes after surgery and continued up to 3 days post-op. Pedia - safety and efficacy in children have not been established CONTRA-INDICATIONS: asthma, urticaria, or allergic-type reactions after taking aspirin or other NSAIDs, history; severe, rarely fatal, anaphylactic-like reactions have been reported, hypersensitivity to diclofenac, aspirin, or other NSAIDs topical gel, use during peri-operative period in setting of coronary artery bypass graft (CABG) surgery; increased risk of myocardial infarction and stroke during the first 10 to 14 days following surgery ADVERSE EFFECTS: Acute renal failure, Agranulocytosis, Anaphylactoid reaction, Anemia, Angioedema, Aplastic anemia, Aseptic meningitis, Blurred vision, Bronchospasm, Cerebrovascular accident, Cirrhosis of liver, Congestive heart failure, Erythema multiforme, Gastrointestinal hemorrhage, Gastrointestinal perforation, Gastrointestinal ulcer, Generalized exfoliative dermatitis, Hearing loss, Hemolytic anemia, Hepatic necrosis, Hepatitis, Hypertension, Inflammatory disorder of digestive tract, Interstitial nephritis, Jaundice, Leukopenia, Liver failure, Melena, Myocardial infarction, Nephrotic syndrome, Pancreatitis, Proteinuria, Purpuric disorder, Seizure, Stevens-Johnson syndrome, Thrombocytopenia, Thrombotic tendency observations, Toxic epidermal necrolysis,Vomiting THERAPEUTIC USE: Antiarrhythmic, Cardiac Glycoside, Cardiovascular Agent, Digitalis Glycoside DOSE: Adult - Congestive heart failure a)(intravenous and/or oral capsule) for rapid digitalization, give loading dose of 0.4 to 0.6 mg ORALLY or IV; additional doses of 0.1 to 0.3 mg ORALLY or IV may be given cautiously at 6 to 8 h intervals if necessary to achieve response (a 70-kg person typically requires 0.6 to 1 mg); for daily maintenance doses or for gradual digitalization, give 0.1 to 0.4 mg ORALLY once daily, titrate every 2 weeks b)(tablet) for rapid digitalization, give loading dose of 0.5 to 0.75 mg ORALLY, additional doses of 0.125 to 0.375 mg ORALLY may be given cautiously at 6 to 8 h intervals if necessary to achieve response (a 70-kg person typically requires 0.75 to 1.25 mg); for daily maintenance doses or for gradual digitalization, give 0.125 to 0.5 mg ORALLY once daily, titrate every 2 weeks. Pedia - Congestive heart failure a)(intravenous and/or oral capsule) for rapid digitalization, give ORALLY or IV in divided doses; (premature) 15 to 25 mcg/kg; (full-term) 21 PRODUCT DESCRIPTION TRADE NAME DOSAGE FORM/STRENGTH Digoxin Antibody Digibind Injection 38mg Dihydrocodeine Tartrate DF 118 Tablet 30mg Injection 25mg Susp. 12mg/mL Tablet 60mg Monotildiem SR SR Tablet 90mg SR Tablet 200mg Tildiem Dilzem Diltiazem Dimenhydrinate Dizinil Tablet 50mg Dramamine PRODUCT INFORMATION 20 to 30 mcg/kg; (1 to 24 mo) 30 to 50 mcg/kg; (2 to 5 y) 25 to 35 mcg/kg; (5 to 10 y) 15 to 30 mcg/kg; (over 10 y) 8 to 12 mcg/kg; for daily maintenance doses or for gradual digitalization, give 20% to 30% of IV digitalizing dose for premature infants or 25% to 35% of oral or IV digitalizing dose for all other pediatric patients b)(elixir) for rapid digitalization, give ORALLY in divided doses; (premature) 20 to 30 mcg/kg; (full-term) 25 to 35 mcg/kg; (1 to 24 mo) 35 to 60 mcg/kg; (2 to 5 y) 30 to 40 mcg/kg; (5 to 10 y) 20 to 35 mcg/kg; (over 10 y) 10 to 15 mcg/kg; for daily maintenance doses or for gradual digitalization, give 20% to 30% of oral digitalizing dose for premature infants or 25% to 35% of oral digitalizing dose for all other pediatric patients c)(solution) older than 2 years; maintenance dose, 10 mcg/kg/day ORALLY, adjust as necessary d)(tablet) daily maintenance dose, give ORALLY in divided doses; (2 to 5 y of age) 10 to 15 mcg/kg; (5 to 10 y of age) 7 to 10 mcg/kg; (over 10 y of age) 3 to 5 mcg/kg CONTRA-INDICATIONS: hypersensitivity to digoxin products, ventricular fibrillation ADVERSE EFFECTS: Cardiac dysrhythmia THERAPEUTIC USE: Digoxin/Digitalis Antidote DOSE: Adult - Digoxin toxicity, a)acute ingestion of unknown amounts, 10 vials (380 mg) IV, observe response; repeat with 10 vials as needed; LIFE THREATENING, 20 VIALS (760 mg) IV b)acute ingestion of known amount digOXIN, each vial (38 mg) IV will bind approximately 0.5 mg digOXIN; bioavailability of digOXIN is 0.8 for 0.25 mg tablets OR 1 for 0.2 Lanoxicaps; use the following formula, dose (in vials) = digOXIN ingested (mg) X bioavailability / 0.5 mg of digOXIN bound per vial c)chronic digOXIN toxicity, 6 vials (228 mg) IV OR use the following formula: dose (in vials) = (serum digOXIN concentration in ng/mL) x (wt in kg)/100. Pedia - Digoxin toxicity, a)acute ingestion of unknown amounts, 10 vials (380 mg); observe response; repeat with 10 vials as needed; LIFE THREATENING, 20 vials (760 mg) IV b)acute ingestion of known amount digOXIN, each vial (38 mg) IV will bind approximately 0.5 mg digOXIN; bioavailability of digOXIN is 0.8 for 0.25 mg tablets OR 1 for 0.2 Lanoxicaps; use the following formula, dose (in vials) = digOXIN ingested (mg) X bioavailability / 0.5 mg of digOXIN bound per vial c)chronic digOXIN toxicity, (infants and small children) single vial (38 mg) IV initially OR use the following formula: number of vials needed = (serum digOXIN concentration in ng/mL) x (wt in kg)/100, then dose (in mg) = number of vials X 38 mg per vial CONTRA-INDICATIONS:none known THERAPEUTIC USE: Opioid DOSE: Adult - one to three tablets (10 to 30 milligrams) three times daily for treatment of COUGH. Smaller doses may also be effective and up to 50 milligrams three times daily is possible. Pedia - aged 6 to 12 years old is 5 to 10 milligrams one to three times daily; aged 2 to 5 years of age is 2.5 to 5 milligrams one to three times daily CONTRA-INDICATIONS: Disease states where respiratory depression would compromise the patient, Long term use in patients with chronic constipation, Sustained-release capsules in patients younger than 12 years of age, Hypersensitivity to dihydrocodeine or other opioids THERAPEUTIC USE: Antianginal, Antihypertensive, Benzothiazepine, Calcium Channel Blocker, Cardiovascular Agent DOSE: Adult – Hypertension a)(sustained release) initial 60-120 mg ORALLY twice daily; usual dose 120-180 mg twice daily, MAX 360 mg/day b)(extended release, Cap) initial 120-240 mg ORALLY once daily; titrate after 14 days; usual dose, 240-360 mg ORALLY once daily, MAX 540 mg/day c)(extended release Tab) initial 180-240 mg ORALLY once daily; dose range 120-540 once daily, MAX 540 mg/day. Pedia not FDA-approved in pediatric patients, Hypertension (TAB, regular release): 1.5-2 mg/kg ORALLY daily in 3-4 divided doses; MAX 3.5 mg/kg daily has been used CONTRA-INDICATIONS: acute MI with pulmonary congestion on x-ray, administration of IV beta-blockers within a few hours of IV diltiazem, atrial fibrillation or flutter associated with an accessory bypass tract (Wolff-Parkinson-White or short PR syndromes); risk of potentially fatal heart rate fluctuations, cardiogenic shock, heart block, second or third-degree atrioventricular without a functioning ventricular pacemaker, hypersensitivity to diltiazem, hypotension, symptomatic, newborns; some injections contain benzyl alcohol, sick sinus syndrome without a functioning ventricular pacemaker, ventricular tachycardia; may lead to hemodynamic deterioration and ventricular fibrillation ADVERSE EFFECTS: Cardiac dysrhythmia THERAPEUTIC USE: Antiemetic, Antihistamine, Antivertigo, Ethanolamine (class), Respiratory Agent DOSE: Adult - Motion sickness; Treatment and Prophylaxis a)50-100 mg ORALLY 30 min prior to travel; repeat every 4-6hrs; maximum dose: 400 mg/day b)50-100 mg IM every 4-6 hrs as needed c)50 mg IV over 2 min (dilute in 10 mL NS before giving). Pedia - not for use in children less than 2 years of age, Motion sickness; Treatment and Prophylaxis a)(2-6 yrs) 12.5-25 mg ORALLY every 6-8 hrs; maximum dose: 75 mg/day b)(6-12 yrs) 25-50 mg ORALLY every 6-8 hrs; maximum dose: 150 mg/day c)1.25 mg/kg or 37.5 mg/m(2) IM every 6 hrs; maximum dose: 300 mg/day CONTRA-INDICATIONS: hypersensitivity to dimenhydrinate or diphenhydramine 22 PRODUCT DESCRIPTION Dimethindine Maleate Dinoprostone TRADE NAME Fenistil Propess Prostin E2 DOSAGE FORM/STRENGTH Drops 1mg/mL Syrup 0.1mg/mL Inj. 10mg/mL Vag. Pessary 10mg Vaginal Tablet 3mg Diosmin/Hesperidine Daflon Tablet 500mg Diphenhydramine HCl Exylin Pedia Syrup 7mg/5mL Diphtheria Pertussis Tetanus & Type B Vaccine Tetract-Hib Injection Diphtheria Tetanus Vaccine D.T. Vax Injection Adult Injection Pedia Dipivefrine HCl Propine Eye Drops 0.1% PRODUCT INFORMATION THERAPEUTIC USE: Alkylamine, Antihistamine, Less-Sedating DOSE: Adult - Immediate Release Tablet, is 1 to 2 milligrams three times daily; Syrup- 1 tsp.(5 mL) of a 0.123-mg/mL syrup up to 9 times daily. Pedia – Drops: a)The usual dose for infants is 0.25 mg (or 5 drops of a 1 mg/mL solution) three times daily. Dimethindene is contraindicated in infants under one month old and should be used cautiously in infants under one year, due to an increased risk of sleep apnea b)children 1 to 8 years old is 0.5 to 0.75 mg (or 10 to 15 drops of a 1 mg/mL solution) three times daily c)children 9 years old and older is 1 mg (20 drops of a 1 mg/mL solution) three times daily. CONTRA-INDICATIONS: Hypersensitivity, Premature infants and neonates, Lactation, Prostate hypertrophy, Bladder obstruction, Hypersensitivity to parabens (contained in the topical gel, oral drops and oral syrup), Cardiac arrhythmias, Alcohol abuse THERAPEUTIC USE: Endocrine-Metabolic Agent, Prostaglandin, Uterine Stimulant DOSE: Adult – Abortion, insert 20 mg suppository high into the vagina; patient to remain supine for 10 min; dose may be repeated at 3- to 5h intervals until abortion occurs; Cervical ripening procedure - Induction of labor a)insert the contents of one syringe (0.5 mg) into the cervical canal just below the level of the internal os; patient to remain in the supine position for at least 15-30 min; repeat dose of 0.5 mg may be given after 6 hours; MAX 1.5 mg/24 hr b)Vaginal Insert: 10 mg placed transversely in the posterior fornix of the vagina; patient to remain in the recumbent position for 2 h following insertion; remove upon onset of active labor or 12 h after insertion. Pedia - Safety and effectiveness in children not established, however, safety and efficacy in adolescents are expected to be the same as in adult women (vaginal insert) CONTRA-INDICATIONS: active cardiac, pulmonary, renal, or hepatic disease, acute pelvic inflammatory disease, cephalopelvic disproportion - suspected or definite, fetal distress - suspected or definite, hypersensitivity to prostraglandins or constituents of the products, multipara with 6 or more previous term pregnancies, previous cesarean section or major uterine surgery, simultaneous IV oxytocic drugs, term pregnancy, unexplained vaginal bleeding during pregnancy ADVERSE EFFECTS: Decreased diastolic arterial pressure, Fetal distress, Fetus or newborn affected by hypertonic labor, Hypertonic uterine dysfunction, Without fetal distress, Myocardial infarction, Rupture of uterus THERAPEUTIC USE: Capillary Hemorrhage, Diabetes mellitus adjunct, Heavy legs, Hemorrhoids, Intermenstrual spotting due to IUD, lymphedema, peripheral venous insufficiency, premenstrual syndrome, stasis ulcer, thromboembolic disorder post-operative prophylaxis. Dose: Adult - Thromboembolic disorder, Postoperative; Prophylaxis ,1000 mg (two 450mg/50mg tablets) every 8 hours the day before surgery, 1000mg 6 hours before surgery, and 1000 mg daily on postoperative days 4 to 15 was administered in combination with lowmolecular-weight heparin CONTRA-INDICATIONS: Hypersensitivity to diosmin THERAPEUTIC USE: Analgesic, Antihistamine Ethanolamine/Acetaminophen Combination, Antipruritic, Antitussive/Expectorant Combination, Antivertigo, Ethanolamine (class), Respiratory Agent, Sleep Aid DOSE: Adult - Allergic rhinitis, 25 to 50 mg ORALLY every 4 to 6 h; maximum dose: 300 mg/day or 10 to 50 mg IM/IV every 2 to 3 h; maximum parenteral dose: 400 mg/day. Pedia - Not recommended in infants or neonates. Allergic rhinitisa)(up to 6 y) 6.25 to 12.5 mg ORALLY every 4 to 6 h b)(6 to 12 y) 12.5 to 25 mg ORALLY every 4 to 6 h; maximum dose: 150 mg/day c)(12 y and older) 25 to 50 mg ORALLY every 4 to 6 h; maximum dose: 300 mg/day d)5 mg/kg/day or 150 mg/m(2)/day IM/IV divided in 4 doses, MAX 300 mg/day CONTRA-INDICATIONS: hypersensitivity to diphenhydramine, newborns or premature infants, nursing mothers ADVERSE EFFECTS: Anaphylaxis THERAPEUTIC USE: Indicated for active immunization against Diphtheria, tetanus, pertussis, Hepatitis B & diseases caused by H. Influenzae type B of all infants from the age og 6weeks onwards. DOSE: Recommended dose 0.5mL of the vaccine must be administered. Primary vaccination schedule consists of three doses to be administered at intervals of at least 4weeks within the first 6months of life. The first dose can be administered at 6weeks of age. CONTRA-INDICATIONS: Hypersensitivity to any component of the vaccine, encephalopathy. ADVERSE EFFECTS: Redness, swelling and pain on the site of injection. Fever, irritability, unusual crying, drowsiness, feeding problems, diarrhea and vomiting. THERAPEUTIC USE: Vaccine DOSE: Pedia – (6 wks to less than 7 y of age) primary series and booster, 0.5mL IM x 5 doses; doses given at 2, 4, and 6 mo of age, and boosters at 15 to 20 mo and at 4 to 6 y of age; recommended intervals: between first 3 doses is 8 wks (4 wk minimum), between dose 3 and 4 is 6 to 12 mo, dose 5 before entry into kindergarten or elementary school (but is not needed if dose 4 was given after 4 th birthday) CONTRA-INDICATIONS: encephalopathy within 7 days of previous pertussis-containing vaccine, hypersensitivity or serious allergic reaction to any vaccine component, history of gelatin or thimerosal allergy, progressive neurologic disorder (eg. Infantile spasm, uncontrolled epilepsy, progressive encephalopathy); defer vaccination until neurologic status is clarified and stabilized ADVERSE EFFECTS: Anaphylaxis, Crying, Persistent, Fever, Seizure THERAPEUTIC USE: Adrenergic, Alkylarylamine, Antiglaucoma, Sympathomimetic DOSE: Adult – Open-angle glaucoma, 1 drop of 0.1% solution TOPICALLY in affected eye(s) every 12 h. Pedia – safety and effectiveness 23 PRODUCT DESCRIPTION TRADE NAME DOSAGE FORM/STRENGTH Dipyridamole Persantin Inj. 5mg/mL Tablet 25mg Tablet 75mg Dobutamine HCl Dobutrex Injection 250mg Domperidone Dompy Tablet 10mg Motilium Dopamine HCl Intropin Inj. 200mg/5mL Dorzolamide Trusopt Eye Drops 2% Doxapram HCl Dopram Inj. 20mg/mL Doxorubicin Adriblastina Inj. 10mg/5mL Inj. 50mg/25mL Doxycycline Hyclate Doxycin Doxydar Vibramycin Capsule 100mg PRODUCT INFORMATION have not been established in pediatric patients CONTRA-INDICATIONS: hypersensitivity to dipivefrin, narrow-angle glaucoma ADVERSE EFFECTS: Cardiac dysrhythmia, Follicular conjunctivitis, Hypertension, Tachyarrhythmia THERAPEUTIC USE: Diagnostic Agent, Cardiac Function, Phosphodiesterase Inhibitor, Platelet Aggregation Inhibitor DOSE: Adult – Heart valve replacement – Thromboembolic disorder; Prophylaxis 75-100 mg ORALLY 4 times daily as an adjunct to warfarin therapy; Radionuclide myocardial perfusion study - 0.142 mg/kg/min IV for 4 min (0.57 mg/kg total) prior to thallium; maximum 60 mg. Pedia – safety and effectiveness in the pediatric population have not been established CONTRA-INDICATIONS: hypersensitivity to dipyridamole products and any components of the product ADVERSE EFFECTS: Angina, Bronchospasm, Myocardial infarction,Ventricular arrhythmia THERAPEUTIC USE: Adrenergic, Vasopressor DOSE: Adult – Decreased cardiac output & Heart failure initial, 0.5-1 mcg/kg/min IV; maintenance, 2.5-20 mcg/kg/min IV; titrate according to response; MAX dose, 40 mcg/kg/min IV. Pedia – not FDA approved in children, Decreased cardiac output 2-20 mcg/kg/min IV; titrate according to response; MAX dose, 40 mcg/kg/min IV CONTRA-INDICATIONS: hypersensitivity to dobutamine, idiopathic hypertrophic subaortic stenosis ADVERSE EFFECTS: Cardiac dysrhythmia, Eosinophilic myocarditis, Thrombocytopenia THERAPEUTIC USE: Antiemetic, Dopamine Antagonist DOSE: Adult – Indigestion, Postprandial Usual dose, 10 to 20 milligrams up to three times daily, before meals and at night, symptomatically. Pedia - Usual dose, 200 to 400 micrograms per kilogram body-weight every 4 to 8 hours CONTRA-INDICATIONS: Hypersensitivity to domperidone THERAPEUTIC USE: Adrenergic, Vasopressor DOSE: Adult – initial, 2 to 5 mcg/kg/min IV; increase in 5 to 10 mcg/kg/min increments; MAX 50 mcg/kg/min IV. Pedia – not FDA approved in children. Hypotension, acute, initial, 2 to 5 mcg/kg/min IV; increase in 5 to 10 mcg/kg/min increments; MAX 30 mcg/kg/min IV CONTRA-INDICATIONS: hypersensitivity to dopamine, pheochromocytoma, tachyarrhythmias/ventricular fibrillation ADVERSE EFFECTS: Ectopic beats, Gangrenous disorder, Ventricular arrhythmia, Wide QRS complex THERAPEUTIC USE: Antiglaucoma, Carbonic Anhydrase Inhibitor DOSE: Adult – Raised intraocular pressure, In patients with ocular hypertension or open-angle glaucoma, 1 drop TOPICALLY in affected eye(s) 3 times daily. Pedia – safety and effectiveness not established in pediatric patients CONTRA-INDICATIONS: hypersensitivity to dorzolamide products ADVERSE EFFECTS: Immune hypersensitivity reaction, Ocular, Iridocyclitis, Rash, Urolithiasis THERAPEUTIC USE: Stimulant, Respiratory DOSE: Adult – Chronic obstructive pulmonary disease – Hypercapnia, acute, 1 to 2 mg/min IV continuous infusion (0.5 to 1 ml/min of a 2 mg/mL solution), may increase to 3 mg/min as needed; MAX infusion time 2 h. Pedia – not FDA approved in children under 12 yr of age; Apnea of prematurity 0.5 to 2.5 mg/kg/h IV infusion CONTRA-INDICATIONS: cardiovascular disorders, doxapram formulation available in the US contains benzyl alcohol; it is contraindicated in newborns, epilepsy or convulsive disorder, head injury or cerebral vascular accident, hypersensitivity to doxapram products, mechanical ventilatory disorders, severe hypertension ADVERSE EFFECTS: Cardiac dysrhythmia, Chest pain, Dyspnea, Hemolysis, Thrombophlebitis, Wheezing THERAPEUTIC USE: Anthracycline, Antineoplastic Agent DOSE: Adult & Pedia – Acute lymphoid leukemia a)(single agent): 60 to 75 mg/m(2) IV every 21 days b)(in combination with other chemotherapy agents): 40 to 60 mg/m(2) IV every 21 to 28 days. CONTRA-INDICATIONS: myelosuppression, previous complete cumulative doses of doxorubicin, daunorubicin, idarubicin, and other anthracyclines and anthracenes ADVERSE EFFECTS: Cardiac dysrhythmia, Congestive heart failure, Myelosuppression THERAPEUTIC USE: Amebicide Intestinal, Antiacne, Antibacterial, Antibiotic, Antimalarial, Antiprotozoal, Dental Agent, Tetracycline (class) DOSE: Adult – Acinetobacter infection, 100 mg ORALLY every 12 hr on day 1 then 100 mg/day ORALLY in 1-2 divided doses; severe infections, 100 mg ORALLY every 12 hours. Pedia - over 8 years, (under 45 kg) 4.4 mg/kg ORALLY in 1-2 divided doses on day 1 then 2.24.4 mg/kg/day ORALLY in 1-2 divided doses; (over 8 years, over 45 kg) use adult dose CONTRA-INDICATIONS: hypersensitivity to doxycycline/tetracycline products ADVERSE EFFECTS: Bulging fontanelle 24 PRODUCT DESCRIPTION TRADE NAME DOSAGE FORM/STRENGTH Dydrogesterone Duphaston Tablet 10mg Edrophonium Tensilon Inj. 10mg/mL Enalapril Maleate Enoxaparin Sodium Renitec Riapril Clexane Ephedrine HCl Syrup 0.1mg/mL Tablet 10mg Injection 2000iu Injection 4000iu Injection 8000iu Inj. 50mg/mL Epirubicin HCl Farmorubicin Inj. 10mg/5mL Inj. 50mg/25mL Epoetin Alfa Eprex Injection 1000iu Injection 4000iu PRODUCT INFORMATION THERAPEUTIC USE: Treatment of progesterone deficiencies such as: dysmenorrheal, endometriosis, secondary amenorrhea, irregular cycles, functional bleeding, premenstrual syndrome, threatened and habitual abortion associated with proven progesterone deficiency, infertility due to luteal insufficiency. To counteract the effects of unopposed estrogen on the endometrium (HRT). DOSE: Adult – Varies from 10 mg to 40 mg per day and depends on the indication for which it is prescribed. THERAPEUTIC USE: Cholinesterase Inhibitor, Diagnostic Agent, Myasthenia Gravis Nondepolarizing Muscle Relaxant Antagonist DOSE: Adult – Myasthenia gravis; Diagnosis a)2 mg IV over 15 to 30 seconds, if no reaction in 45 seconds, give additional 8 mg b)in adults with inaccessible veins, 10 mg IM. Pedia – Myasthenia gravis; a)(infants) 0.5 mg IV or 0.5 to 1 mg IM or SC b)(34 kg or less) 1 mg IV, if no reaction in 45 seconds, may repeat at a rate of 1 mg every 30 to 45 seconds to maximum cumulative dose of 5 mg or 2 mg IM c)(over 34 kg) 2 mg IV, if no reaction in 45 seconds, may repeat at a rate of 1 mg every 30 to 45 seconds to maximum cumulative dose of 10 mg or 5 mg IM CONTRA-INDICATIONS: hypersensitivity to edrophonium chloride or anticholinesterase agents, hypersensitivity to sulfite agents, intestinal or urinary obstruction of the mechanical type ADVERSE EFFECTS: Bronchospasm, Cardiac arrest, Respiratory tract paralysis THERAPEUTIC USE: ACE Inhibitor, Antihypertensive, Cardiovascular Agent, Renal Protective Agent DOSE: Adult – Heart failure, initial, 2.5 mg ORALLY once or twice daily; maintenance, 2.5-20 mg ORALLY twice daily; MAX 20 mg daily in divided doses. Pedia – Hypertension 6 to 16 years: initial, 0.08 mg/kg ORALLY once daily (up to 5 mg), MAX 0.58 mg/kg or 40 mg daily CONTRA-INDICATIONS: ACE-inhibitor induced angioedema, hereditary or idiopathic angioedema, hypersensitivity to enalapril/other ACE inhibitors, pregnancy ADVERSE EFFECTS: Angioedema, Intestinal angioedema, Liver failure, Syncope THERAPEUTIC USE: Anticoagulant, Low Molecular Weight Heparin DOSE: Adult – 40 mg SUBQ every 24 hours; give initial dose 2 hours prior to surgery and continue for 7 to 10 days. Pedia – Not FDA approved in children CONTRA-INDICATIONS: active major bleeding, hypersensitivity to enoxaparin, heparin, pork products, hypersensitivity to benzyl alcohol, thrombocytopenia associated with a positive test for antiplatelet antibody in the presence of enoxaparin. ADVERSE EFFECTS: Anaphylactoid reaction, Atrial fibrillation, Eczematous drug eruption, Heart failure, Hematoma, Spinal, Hemorrhage, Major, Increased liver function test, Pneumonia, Pulmonary edema, Skin necrosis, Thrombocytopenia THERAPEUTIC USE: Adrenergic, Alkylarylamine, Bronchodilator, CNS Stimulant, Decongestant,, Sympathomimetic, Vasopressor DOSE: Adult - Complication of anesthesia - Drug-induced hypotension usual dose, 25 to 50 mg (range, 10 to 50 mg) injected SUBQ/IM/IV; Nasal congestion, 25 to 50 mg ORALLY every 6 h as needed; 0.5% solution or 0.6% jelly Intranasally every 4 h CONTRA-INDICATIONS: anesthesia with cyclopropane or halothane, diabetes hypersensitivity to ephedrine/sympathomimetic amines, hypertension or other cardiovascular disorders, pregnancy with maternal blood pressure above 130/80, thyrotoxicosis THERAPEUTIC USE: Anthracycline, Antineoplastic Agent DOSE: Adult – Breast cancer, Adjuvant therapy for axillary node-positive disease a)initial starting dose, 100 to 120 mg/m(2) IV given every 3 to 4 weeks b)60 mg/m(2) IV on days 1 and 8 repeated every 28 days for 6 cycles in combination with cyclophosphamide and 5-fluorouracil c)100 mg/m(2) on day 1 repeated every 21 days for 6 cycles in combination with cyclophosphamide and 5-fluorouracil. Pedia – safety & efficacy have not been established in pediatric patients. CONTRA-INDICATIONS: hypersensitivity to anthracenediones, hypersensitivity to epirubicin or other anthracyclines, neutrophil count less than 1500 cells/mm(3), prior cumulative max dose of anthracyclines, severe hepatic dysfunction, severe myocardial insufficiency, recent MI, severe arrhythmias. ADVERSE EFFECTS: Abnormal ECG, Acute myeloid leukemia, secondary, Anaphylaxis, Cardiotoxicity, Possibly fatal CHF, Hyperuricemia, Immune hypersensitivity reaction, Myelosuppression, Possibly severe, Tissue necrosis, Local, Tumor lysis syndrome THERAPEUTIC USE: Erythropoietic, Hematopoietic DOSE: Adult – Anemia in neoplastic disease, due to chemotherapy (non-myeloid malignancy) a)(three times per week dosing) 150 units/kg subcutaneously 3 times per week; if no response after 8 weeks, may increase to 300 units/kg 3 times per week; discontinue after the completion of the chemotherapy course b)(once weekly dosing) 40,000 units subcutaneously once weekly; if no response after 4 weeks, may increase to 60,000 units subcutaneously once weekly; discontinue after the completion of the chemotherapy course. Pedia – safety and efficacy of epoetin alfa has not been established in pediatric patients less than 1 month old with chronic renal failure who are on dialysis; Anemia in neoplastic disease, due to chemotherapy (non-myeloid malignancy), 600 units/kg (MAX, 40,000 units) IV once weekly; if no response after 4 weeks, may increase to 900 units/kg (MAX, 60,000 units) IV weekly; discontinue after the completion of the chemotherapy course CONTRA-INDICATIONS: hypersensitivity to human albumin, hypersensitivity to mammalian cell-derived products, hypertension, uncontrolled; hypertensive encephalopathy and seizures have been observed 25 PRODUCT DESCRIPTION TRADE NAME Erythrodar Eye Oint. 5mg/g Susp. 200mg/5mL Tablet 250mg Erythromycin Lactobionate Erythrocin Erythrodar Injection 500mg Injection 1gram Esmolol HCl Brevibloc Inj. 10mg/mL Ethambutol Myambutol Tablet 100mg Tablet 400mg Erythromycin Erythrocin DOSAGE FORM/STRENGTH Ethanolamine Injection 5% Etoposide VP – 16 Inj. 100mg/5mL Fat Emulsion Lipofundin Inj. 20%, 100mL Inj. 20%, 250mL Inj. 20%, 500mL PRODUCT INFORMATION ADVERSE EFFECTS: Cerebrovascular accident, Congestive heart failure, Deep venous thrombosis, Hypertension, Left ventricular failure, acute, Myocardial infarction, Pulmonary embolism, Seizure, Thrombotic disorder, Thrombotic microangiopathy, Thrombus due to any device, implant &/or graft, Transient ischemic attack THERAPEUTIC USE: Amebicide Intestinal, Antiacne, Antibacterial, Antibiotic, Macrolide DOSE: Adult – 250 mg ORALLY every 6 h or 500 mg ORALLY every 12 h; MAX 4 g/day or 250 mg ORALLY every 6 h or 333 mg ORALLY every 8 h or 500 mg ORALLY every 12 h; MAX 4 g/day, depending on type and severity of infection. Pedia – 30 to 50 mg/kg/day ORALLY divided every 6 to 8 h; MAX 4 g/day as base, depending on type and severity of infection. CONTRA-INDICATIONS: concomitant therapy with astemizole, cisapride, pimozide, or terfenadine, hypersensitivity to erythromycin or any component of the product THERAPEUTIC USE: Amebicide Intestinal, Antiacne, Antibacterial, Antibiotic, Macrolide DOSE: Adult & Pedia - 15 to 20 mg/kg/day IV in divided doses; MAX 4 g/day, depending on type and severity of infection. CONTRA-INDICATIONS: concomitant therapy with astemizole, cisapride, pimozide, or terfenadine, hypersensitivity to erythromycin products. THERAPEUTIC USE: Antiarrhythmic, Group II, Beta-Adrenergic Blocker, Cardioselective, Cardiovascular Agent DOSE: Adult – Hypertension, Intraoperative – Tachyarrhythmia, Intraoperative a)(immediate control) initial, 80 mg IV bolus over 30 seconds followed by 150 mcg/kg/min IV infusion b)(immediate control) titration, may titrate up to 300 mcg/kg/min. Pedia – safety and effectiveness not established CONTRA-INDICATIONS: cardiogenic shock, hypersensitivity to esmolol, overt cardiac failure, second and third degree AV block, severe sinus bradycardia ADVERSE EFFECTS: Bronchospasm, Pulmonary edema, Seizure THERAPEUTIC USE: Antitubercular DOSE: Adult – should not be used alone as initial or retreatment, Pulmonary tuberculosis; Adjunct a)initial, 15 mg/kg ORALLY once daily as combination therapy with other antitubercular agents (such as isoniazid, rifampin, pyrazinamide) b)retreatment, 25 mg/kg ORALLY once daily for 60 days then 15 mg/kg ORALLY once daily as combination therapy with other antitubercular agents (such as isoniazid, rifampin, pyrazinamide) c)NOT routinely recommended for preventive therapy for tuberculosis. Pedia – not recommended for children less than 13 y. Pulmonary tuberculosis; Adjunct initial, (over 13 y) 15 mg/kg ORALLY once daily as combination therapy with other antitubercular agents. CONTRA-INDICATIONS: hypersensitivity to ethambutol products, patients with known optic neuritis, unless clinical judgment determines that it may be used, patients unable to appreciate and report visual side effects or changes in vision. ADVERSE EFFECTS: Anaphylactoid reaction, Blindness &/or vision impairment level, Neutropenia, Optic neuritis, Peripheral neuropathy, Thrombocytopenia THERAPEUTIC USE: Sclerosing Agent DOSE: Adult – Bleeding esophageal varices, 1.5 to 5 mL per varix IV at the time of the acute bleeding episode and then after 1 week, 6 weeks, 3 months, and 6 months as indicated; MAX dose per treatment session, 20 mL. Pedia- safety & efficacy not established in children CONTRA-INDICATIONS: hypersensitivity to ethanolamine oleate, oleic acid, or ethanolamine ADVERSE EFFECTS: Acute renal failure, Anaphylaxis, Esophageal perforation, Esophageal stricture, Pulmonary embolism THERAPEUTIC USE: Antineoplastic Agent, Mitotic Inhibitor DOSE: Adult – Small cell carcinoma of lung, in combination therapy with other approved chemotherapeutic agents as first-line therapy a)Range, 35 mg/m(2)/day IV for 4 days, to 50 mg/m(2)/day IV for 5 days; in combination with other approved chemotherapeutic agents; repeat at 3-4 week intervals b)ORAL dose is 2 times the IV dose rounded to the nearest 50 mg. Pedia - safety and effectiveness in children have not been established CONTRA-INDICATIONS: hypersensitivity to podophyllum ADVERSE EFFECTS: Acute leukemia, Congestive heart failure, Hepatotoxicity, Immune hypersensitivity reaction, Myelosuppression, Doselimiting, Myocardial infarction, Stevens-Johnson syndrome, Toxic epidermal necrolysis THERAPEUTIC USE: Parenteral Lipids DOSE: Adult – Total parenteral nutrition, doses should be individualized based upon the caloric requirements of the patient; daily dose should not exceed 2.5g fat/kg of body weight and should not exceed 60% of the total daily caloric input. Pedia – Total parenteral nutrition a)older patients: doses should be individualized based upon the caloric requirements of the patient; daily dose should not exceed 3 g fat/kg of body weight and should not exceed 60% of the total daily caloric input b)premature infants, doses should be individualized based upon the caloric requirements of the patient; suggested initial dosage, 0.5 g fat/kg of body weight per 24 h IV and may be increased in relation to the infants' ability to eliminate fat up to a maximum of 3 g fat /kg per 24 h; rate should not exceed 1 g fat/kg in 4 h CONTRA-INDICATIONS: abnormal fat metabolism, including pathological hyperlipidemia, lipid nephrosis, or acute pancreatitis with hyperlipidemia ADVERSE EFFECTS: Cholestasis, Dyspnea, Hepatomegaly, Pulmonary fat embolism, Splenomegaly, Thrombocytopenia 26 PRODUCT DESCRIPTION Fentanyl TRADE NAME Durogesic Sublimase DOSAGE FORM/STRENGTH Inj. 0.1mg/2mL Inj. 500mcg/10mL Patch 25mcg/Hr Patch 50mcg/Hr Ferric Hydroxide Polymaltose Ferose Ferrous Fumarate Fumafer Tablet 200mg Ferrous Sulphate Feromin Tablet 190mg Fertonic Fibrinogen Drops 25mg Iron Syrup 50mg Iron Injection 1gram Filgrastim Neupogen Inj. 300mcg/mL Flucloxacillin Floxapen Capsule 500mg Injection 500mg PRODUCT INFORMATION THERAPEUTIC USE: Analgesic, Anesthetic Adjunct, Opioid DOSE: Adult - transdermal system: only for use in the management of chronic, moderate to severe pain requiring around-the-clock opioid therapy not managed by other means in patients who have demonstrated opioid tolerance and require a total daily dose equivalent to the fentanyl 25 mcg/hr transdermal patch; management of acute or post-operative pain in opiate-naive patients is contraindicated. Pedia - the safety of the fentanyl iontophoretic transdermal system has not been established in children CONTRA-INDICATIONS: acute or postoperative pain; risk of life-threatening respiratory depression/hypoventilation, bronchial asthma, acute or severe; risk of serious or life-threatening hypoventilation, hypersensitivity to fentanyl or any product or system components, mild or intermittent pain management; risk of serious or life-threatening hypoventilation, opioid nontolerant patients; risk of life-threatening respiratory depression, paralytic ileus, suspected or known, situations of significant respiratory depression and unmonitored settings that lack resuscitative equipment ADVERSE EFFECTS: Apnea, Cardiac dysrhythmia, Chest pain, Hypertension, Hypotension, Hypoventilation, Respiratory depression THERAPEUTIC USE: Iron Supplement, Nutriceutical, Parenteral Mineral-Trace Mineral DOSE: Adult – Iron deficiency anemia, ORAL, elemental iron 2-3 mg/kg DAILY (divided into 3 doses). Pedia – Iron deficiency anemia a)(premature infants) 2 to 4 mg elemental iron/kg/day ORALLY (divided into 1 or 2 doses), MAX 15 mg/day b)(infants and children) 3 to 6 mg elemental iron/kg/day ORALLY (divided into 1 to 3 doses) CONTRA-INDICATIONS: anemia other than iron-deficiency anemia, hemochromatosis, hemosiderosis, hypersensitivity to iron products ADVERSE EFFECTS: Immune hypersensitivity reaction THERAPEUTIC USE: Iron Supplement, Nutriceutical, Parenteral Mineral-Trace Mineral DOSE: Adult – Iron deficiency anemia, ORAL, elemental iron 2-3 mg/kg DAILY (divided into 3 doses). Pedia – Iron deficiency anemia a)(premature infants) 2 to 4 mg elemental iron/kg/day ORALLY (divided into 1 or 2 doses), MAX 15 mg/day b)(infants and children) 3 to 6 mg elemental iron/kg/day ORALLY (divided into 1 to 3 doses) CONTRA-INDICATIONS: anemia other than iron-deficiency anemia, hemochromatosis, hemosiderosis, hypersensitivity to iron products ADVERSE EFFECTS: Immune hypersensitivity reaction THERAPEUTIC USE: Iron Supplement, Nutriceutical, Parenteral Mineral-Trace Mineral DOSE: Adult – Iron deficiency anemia, ORAL, elemental iron 2-3 mg/kg DAILY (divided into 3 doses). Pedia – Iron deficiency anemia a)(premature infants) 2 to 4 mg elemental iron/kg/day ORALLY (divided into 1 or 2 doses), MAX 15 mg/day b)(infants and children) 3 to 6 mg elemental iron/kg/day ORALLY (divided into 1 to 3 doses) CONTRA-INDICATIONS: anemia other than iron-deficiency anemia, hemochromatosis, hemosiderosis, hypersensitivity to iron products ADVERSE EFFECTS: Immune hypersensitivity reaction THERAPEUTIC USE: To control haemorrhage associated with low blood-fibrinogen concentration in afibrinogenaemia or hypofirbrinogenaemia. It has also been used in disseminated intravascular coagulation. DOSE: Adult - Generally, 1-2 grams is administered initially, with subsequent infusions as required. Pedia – the dosage should be selected according to the body weight of the child and clinical need. CONTRA-INDICATIONS: Hypersensitivity to constituents of the preparation. ADVERSE EFFECTS: Anaphylactic reaction, potential risk of thromboembolic episodes. THERAPEUTIC USE: Colony Stimulating Factor, Hematopoietic DOSE: Adult & Pedia – Febrile neutropenia, In non-myeloid malignancies following myelosuppressive chemotherapy; Prophylaxis 5 mcg/kg/day SC/IV once daily; start at least 24 h after chemotherapy. CONTRA-INDICATIONS: Hypersensitivity to E.coli derived proteins, filgrastim or any component of the product ADVERSE EFFECTS: Acute respiratory distress syndrome, Hemoglobin SS disease with crisis, Rupture of spleen, Vasculitis of the skin THERAPEUTIC USE: Penicillin, Penicillinase-Resistant DOSE: Adult – oral doses of floxacillin have been 250 mg every 6 to 8 hours. However, higher doses (500 to 2000 mg every 8 hours) have been used in some studied; IV dose of floxacillin is 250 to 500 mg every 6 hours. IM, a dose of 250 mg every 6 hours has been suggested. Higher doses are indicated in more severe infections over 3 to 4 minutes. Pedia – oral dose of floxacillin in children 2 to 10 years and under 2 years of age is 125 mg and 62.5 mg, respectively, every 6 hours; children 2 to 10 years of age, IV doses of 125 to 250 mg every 6 hours are suggested. An IM dose of 125 mg every 6 hours has been recommended in this age group. For younger children (under 2 years of age), one-half of the above dose is suggested. CONTRA-INDICATIONS: Hypersensitivity to FLOXACILLIN or other penicillins 27 PRODUCT DESCRIPTION TRADE NAME Diflucan Capsule 50mg Inj. 2mg/mL Syrup 1mg/mL Flucytosine Ancotil Inj. 10mg/mL Fludarabine Phosphate Fludara Injection 50mg Fluconazole Fludrocortisone Acetate Candivast DOSAGE FORM/STRENGTH Cortilon Florinef Syrup 10mcg/mL Tablet 100mcg Flumazenil Anexate Inj. 100mcg/mL Flumethasone/Salicylic Acid Locasalen Ointment Fluorescein Sodium Fluorets Eye Minims 2% Eye Strips 1mg Injection 10% PRODUCT INFORMATION THERAPEUTIC USE: Antifungal, Triazole DOSE: Adult – Candidiasis of the mouth, 200 mg ORALLY or IV on the first day, then 100 mg once daily; treatment should be continued for at least 2 weeks to decrease the likelihood of relapse; Candidiasis of the esophagus, 200 mg ORALLY or IV on the first day, then 100 mg once daily (MAX daily dose 400 mg); treatment should last for at least 3 weeks and for at least 2 weeks following resolution of symptoms. Pedia – Candidiasis of the mouth, 6 mg/kg/day ORALLY or IV on day 1, then 3 mg/kg/day once daily for at least 2 weeks to decrease the likelihood of relapse; Candidiasis of the esophagus, 6 mg/kg/day ORALLY or IV on day 1, then 3 mg/kg/day (MAX 12 mg/kg/day) once daily for at least 3 weeks; continue treatment for 2 weeks following resolution of symptoms CONTRA-INDICATIONS: hypersensitivity to fluconazole ADVERSE EFFECTS: Anaphylaxis THERAPEUTIC USE: Antifungal DOSE: Adult – Candidiasis (septicemia, endocarditis, urinary tract infections, pulmonary infections) 50 to 150 mg/kg/day ORALLY divided into 4 doses at 6-h intervals. Pedia – safety and efficacy in children not established CONTRA-INDICATIONS: hypersensitivity to flucytosine ADVERSE EFFECTS: Cardiotoxicity, Leukopenia, Thrombocytopenia THERAPEUTIC USE: Antimetabolite, Antineoplastic Agent DOSE: Adult – B-cell chronic lymphocytic leukemia, For patients who have not responded to or whose disease has progressed during treatment with at least one standard alkylating-agent containing regimen, 25 mg/m(2) IV daily for 5 days, repeat every 28 days; dosage should be adjusted based on hematologic or non-hematologic toxicity. Pedia – efficacy has not been established in any childhood malignancy CONTRA-INDICATIONS: hypersensitivity to fludarabine products ADVERSE EFFECTS: Aplasia of skin, Autoimmune hemolytic anemia, Edema, Fever, Graft versus host disease, Transfusion-associated, with non-irradiated blood, Infectious disease, Myelosuppression, Neurotoxicity, Pneumonia THERAPEUTIC USE: Adrenal Mineralocorticoid, Diagnostic Agent, Adrenocortical Function, Endocrine-Metabolic Agent DOSE: Adult – Addison's disease – Adrenal insufficiency, 0.1 mg ORALLY 3 times/wk to 0.2 mg/day ORALLY. Pedia – Not FDA approved in children. Addison's disease – Adrenal insufficiency a)infants, 0.1 to 0.2 mg/day ORALLY b)children, 0.05 to 0.1 mg/day ORALLY CONTRA-INDICATIONS: hypersensitivity to fludrocortisones, systemic fungal infections ADVERSE EFFECTS: Cardiomegaly, Congestive heart failure, Hypertension, Raised intracranial pressure, Secondary hypocortisolism, Seizure, Thrombophlebitis THERAPEUTIC USE: Benzodiazepine Antagonist, Toxicology-Antidote Agent DOSE: Adult – Drug overdose, Benzodiazepine, known or suspected initial, 0.2 mg IV over 30 s; if desired level of consciousness not obtained after an additional 30 s, give dose of 0.3 mg IV over 30 s; further doses of 0.5 mg IV over 30 s may be given at 1-min intervals if needed to MAX total dose of 3 mg; patients with only partial response to 3 mg may require additional slow titration to a total dose of 5 mg; if no response 5 min after receiving total dose of 5 mg, overdose is unlikely to be benzodiazepine and further treatment with flumazenil will not help. Pedia – safety and efficacy in the reversal of conscious sedation in pediatric patients below the age of 1 year have not been established. Reversal of benzodiazepine activity, children 1 year or older, 0.01 mg/kg (up to 0.2 mg) IV over 15 seconds; if adequate sedation reversal does not occur after an additional 45 seconds, further injections of 0.01 mg/kg (up to 0.2 mg) may be repeated at 1-minute intervals, as needed up to 4 times; maximum total dose 0.05 mg/kg or 1 mg, whichever is lower CONTRA-INDICATIONS: hypersensitivity to flumazenil or benzodiazepines, patients who are showing signs of serious cyclic antidepressant overdose, patients who have been given a benzodiazepine for control of a potentially life-threatening condition (eg, control of intracranial pressure or status epilepticus). ADVERSE EFFECTS: Cardiac dysrhythmia, Death, Usually in patients with serious underlying disease or who had ingested large amounts of non-benzodiazepine drugs, (usually cyclic antidepressants), as part of an overdose, Seizure, In patients who are relying on benzodiazepines to control seizures, are physically dependent on benzodiazepines, or who have ingested large doses of other drugs THERAPEUTIC USE: Adrenal Glucocorticoid DOSE: Adult & Pedia – Flumethasone cream 0.03% should be applied in a thin film to the affected area 3 to 4 times daily. CONTRA-INDICATIONS: Hypersensitivity to flumethasone or components THERAPEUTIC USE: Disclosing Agent DOSE: Adult - Ophthalmic fluorescence imaging, inject contents of ampul or vial of 10% or 25% solution rapidly IV into the antecubital vein; Applanation tonometry-place strip, which has been moistened with a drop of sterile water, at the fornix in the lower cul-de-sac close to the punctum; patient should close lid tightly over strip until desired amount of staining is observed or retract upper lid and touch tip of strip to the bulbar conjunctiva on the temporal side until adequate staining is achieved. Pedia – Safety & effectiveness not established in children CONTRA-INDICATIONS: concomitant soft contact lens use, hypersensitivity to any component of fluorescein ADVERSE EFFECTS: Anaphylaxis, Injection, Arterial ischemia, Basilar, Cardiac arrest, Injection site thrombophlebitis, Seizure, Shock 28 PRODUCT DESCRIPTION Fluorometholone TRADE NAME FML DOSAGE FORM/STRENGTH Eye Drops 0.1% Inj. 250mg/5mL Inj. 500mg/10mL Fluorouracil Fluticasone Propionate Flixotide Evohaler 125mcg Evohaler 250mcg Folic Acid Folicum Vitafol Inj. 5mg/mL Syrup 1mg/mL Tablet 5mg Fondaparinux Arixtra Inj. 2.5mg/0.5mL Fosinopril Monopril Staril Tablet 10mg Tablet 20mg PRODUCT INFORMATION THERAPEUTIC USE: Adrenal Glucocorticoid, Ophthalmologic Agent DOSE: Adult & Pedia – Inflammatory disorder of the eye a)ophthalmic ointment, initial, apply one-half inch ribbon into the conjunctival sac every 4 hrs b)ophthalmic ointment, maintenance, apply one-half inch ribbon into the conjunctival sac 1 to 3 times daily c)ophthalmic suspension: Instill 1 drop into the conjunctival sac 2 to 4 times daily. CONTRA-INDICATIONS: epithelial herpes simplex keratitis, mycobacterial infections of the eye, fungal diseases of the ocular structures, hypersensitivity to preparation components, vaccinia, varicella, or other viral diseases of the eye ADVERSE EFFECTS: Glaucoma, Perforating scleral wound THERAPEUTIC USE: Antimetabolite, Antineoplastic, Dermatological DOSE: Adult – Carcinoma of pancreas, Palliative 12 mg/kg IV daily for 4 days, 6 mg/kg IV daily on days 6, 8, 10, 12 (maximum dose 800 mg/day); maintenance therapy- may be considered in patients tolerating fluorouracil toxicity by repeating the first course every 30 days, after the last day of the previous treatment cycle. Alternatively, a single maintenance dose of 10-15 mg/kg/week may be given after toxic signs resulting from the initial course of therapy have subsided; maximum dose 1 g/week. Pedia – Safety and effectiveness in children have not been established CONTRA-INIDICATIONS: Bone marrow depression, Dihydropyrimidine dehydrogenase enzyme deficiency, Hypersensitivity to fluorouracil, Poor nutritional state, Pregnancy, existing or potential, Serious infection ADVERSE EFFECTS: Anaphylaxis, Angina, Bleeding, Cerebellar syndrome, acute, Coronary arteriosclerosis, Eye / vision finding, Finding of lacrimation, Gastrointestinal ulcer, Immune hypersensitivity reaction, Light intolerance, Myelosuppression, Anemia, eucopenia, thrombocytopenia, Nystagmus, Stenosis of lacrimal system, Thrombophlebitis THERAPEUTIC USE: Adrenal Glucocorticoid, Anti-Inflammatory, Corticosteroid DOSE: Adult – Allergic rhinitis, Nasal Inhalation, initial, 2 sprays/nostril daily or 1 spray/nostril twice daily, MAX 2 sprays/nostril/day (200 mcg/day). Pedia - Allergic rhinitis Nasal Inhalation, 4 y and older, initial, 1 spray/nostril once daily, MAX 2 sprays/nostril daily (200 mcg/day) CONTRA-INDICATIONS: hypersensitivity to fluticasone or other ingredients, severe allergy to milk proteins, status asthmaticus or other acute episodes of asthma. ADVERSE EFFECTS: Anaphylaxis, Glaucoma, Immune hypersensitivity reaction, Secondary hypocortisolism THERAPEUTIC USE: Nutritive Agent, Vitamin B DOSE: Adult – Folic acid deficiency, up to 1 mg/day IM, IV, ORAL, SC; resistant cases may require larger doses; maintenance, 0.4 mg/day ORALLY; for pregnant and lactating women, 0.8 mg/day ORALLY; never less than 0.1 mg/day. Pedia - ORAL, IM, IV, SC, up to 1 mg/day; resistant cases may require larger doses, maintenance, infants, 0.1 mg/day; age under 4 y, up to 0.3 mg/day; age 4 y or older, 0.4 mg/day CONTRA-INDICATIONS: hypersensitivity to folic acid products ADVERSE EFFECTS: Allergy THERAPEUTIC USE: Anticoagulant, Factor Xa Inhibitor DOSE: Adult – Postoperative deep vein thrombosis, Hip repair or replacement, knee replacement, or abdominal surgery; Prophylaxis 2.5 mg SUBQ once daily after hemostasis has been established (initial dose should be given 6-8 hrs post-op); usual duration of therapy is 5 to 9 days; for hip fracture patients an extended course of up to 24 days is recommended. Pedia – safety and efficacy in pediatrics have not been established CONTRA-INDICATIONS: active major bleeding; risk of uncontrollable hemorrhage, bacterial endocarditis, body weight less than 50 kg for prophylactic therapy of hip fracture, hip replacement or knee replacement surgery, or abdominal surgery; increased risk for major bleeding episodes, fondaparinux-related thrombocytopenia, hypersensitivity to fondaparinux, severe renal impairment (creatinine clearance less than 30 mL/min); increased risk for major bleeding episodes ADVERSE EFFECTS: Anemia, Bleeding, Major, in those greater than 75 years old, Bleeding, Major, in those less than 65 years old, Bleeding, Major, with normal renal function, Bleeding, Major, with severe renal impairment, Increased liver aminotransferase level, Asymptomatic, Thrombocytopenia, Less than 50,000/mm(3) THERAPEUTIC USE: ACE Inhibitor, Antihypertensive, Cardiovascular Agent, Renal Protective Agent DOSE: Adult – Congestive heart failure; Adjunct initial, 5 to 10 mg ORALLY once daily; maintenance, 20 to 40 mg ORALLY daily; MAX 40 mg daily; Hypertension – initial, 10 mg ORALLY once daily; maintenance, 20 to 40 mg ORALLY once daily or in 2 divided doses; MAX 80 mg daily; Myocardial infarction – initial, 5 mg ORALLY once daily; maintenance, 5 to 20 mg ORALLY daily; MAX 20 mg/daily. Pedia – Hypertension, (children ages 6 to 16 years weighing more than 50 kg) 5 to 10 mg ORALLY once daily, MAX 40 mg/day CONTRA-INDICATIONS: hypersensitivity to fosinopril/other ACE inhibitors ADVERSE EFFECTS: Angioedema, Head and Neck, Intestinal angioedema, Liver failure 29 PRODUCT DESCRIPTION TRADE NAME Diusemide Furosemide Lasix Salurin DOSAGE FORM/STRENGTH Inj. 20mg/2mL Inj. 50mg/5mL Syrup 1mg/mL Tablet 40mg Fusidate Sodium Balad Fucidin Fusibact Injection 500mg Ointment 2% Tablet 250mg Fusidic Acid Fucithalmic Eye Drops 1% Ganciclovir Cymevene Injection 500mg Gemcitabine HCl Gemzar Injection 200mg Gemfibrozil Lopid Tablet 600mg Low – Lip Garamycin Gentamicin Gentacin Eye/Ear Drops 0.3% Eye Ointment 0.3% Injection 20mg/2mL PRODUCT INFORMATION THERAPEUTIC USE: Cardiovascular Agent, Diuretic, Loop DOSE: Adult – Congestive heart failure – Edema a)initial, 20 to 40 mg IV over 1 to 2 min; may repeat same dose 2 hours later or may be increased by 20 mg until desired response; this individually determined dose may be given once or twice daily b)initial, 20 to 40 mg IM; may repeat same dose 2 hours later or may be increased by 20 mg until desired response; this individually determined dose may be given once or twice daily c)initial, 20 to 80 mg ORALLY daily, may repeat in 6 to 8 hours; MAX 600 mg/day ORALLY d)titrate to maintenance, may increase by 20 to 40 mg ORALLY at 6 to 8 hours intervals after the previous dose; this individual dose may be given once or twice daily; MAX 600 mg/day ORALLY e)IV infusion, administer at a rate not greater than 4 mg/min. Pedia - a)premature neonates less than 29 wk gestation, 1 to 2 mg/kg/dose ORALLY every 24 hours; may increase to 6 mg/kg/dose b)premature neonates less than 29 wk gestation, 1 mg/kg/dose IV every 24 hours c)neonates, 1 to 3 mg/kg ORALLY every 8 hours as needed d)neonates, 1 to 3 mg/kg IV over 3 min; may repeat same dose every 8 hours e)infants and children, initial, 2 mg/kg/dose ORALLY; may increase by 1 to 2 mg/kg/dose no sooner than 6 to 8 hours following the previous dose. MAX 6 mg/kg/dose ORALLY CONTRA-INDICATIONS: hypersensitivity to furosemide or sulfonamides, anuria ADVERSE EFFECTS: Agranulocytosis, Aplastic anemia, Erythema multiforme, Hemolytic anemia, Hypotension, Leukopenia, Pancreatitis, Scaling eczema, Stevens-Johnson syndrome, Thrombocytopenia THERAPEUTIC USE: Infection of skin & soft tissue, infective conjunctivitis, osteomyelitis DOSE: Adult – IV dose in adults is 500 mg sodium fusidate (equivalent 480 mg fusidic acid) 3 times daily, with doses not to exceed 2 gm daily; Pedia – IV dose in children is 20 mg sodium fusidate/kg/day, equivalent to 19.2 mg/kg/day fusidic acid, divided into 3 equal doses and infused over a 2 to 4 hour period into a wide bore vein with good blood flow THERAPEUTIC USE: Infection of skin & soft tissue, infective conjunctivitis, osteomyelitis DOSE: Adult & Pedia – For skin and soft tissue infections the minimum duration of therapy should be 1 to 2 weeks, for acute osteomyelitis a minimum of 2 to 4 weeks, and chronic osteomyelitis several months of therapy may be required. CONTRA-INDICATIONS: Hypersensitivity to fusidic acid and/or its salts THERAPEUTIC USE: Antiviral, Guanosine Nucleoside Analog, Viral DNA Polymerase Inhibitor DOSE: Adult – Cytomegalovirus infection, Transplantation; Prophylaxis a)5 mg/kg IV over 1 hr every 12 hr for 7-14 days, then 5 mg/kg IV once a day 7 days a week, or 6 mg/kg IV once a day 5 days a week; duration of therapy dependent on degree and duration of immunosuppression b)solid organ transplant, 1 g ORALLY 3 times a day. Pedia – not FDA-approved for pediatric patients; use with caution in this population due to the probability of long-term carcinogenicity and reproductive toxicity. Cytomegalovirus infection; Prophylaxis – HIV infection, Advanced a)(primary prophylaxis) 30 mg/kg ORALLY 3 times a day b)(secondary prophylaxis) 5 mg/kg IV once daily CONTRA-INDICATIONS: hypersensitivity to ganciclovir/acyclovir products, patients with severe neutropenia (ANC less than 500/microliter) or severe thrombocytopenia (platelets less than 25,000/microliter), intravitreal implant; any contraindication for ocular surgery including infection or severe thrombocytopenia THERAPEUTIC USE: Antimetabolite, Antineoplastic Agent DOSE: Adult – Carcinoma of pancreas, As first line therapy in locally advanced (nonresectable Stage II or Stage III) or metastatic (Stage IV) disease, or for patients previously treated with 5-fluorouracil a)initial, 1000 mg/m(2) IV over 30 min weekly for 7 wk followed by 1 wk rest b)subsequent, 1000 mg/m(2) IV weekly for 3 consecutive weeks out of every 4 weeks. Pedia - safety and effectiveness in children have not been established CONTRA-INDICATIONS: hypersensitivity to gemcitabine ADVERSE EFFECTS: Acute respiratory distress syndrome, Cardiac dysrhythmia, Congestive heart failure, Hemolytic uremic syndrome, Hepatotoxicity, Hypertension, Interstitial pneumonia, Liver failure, Myelosuppression, Dose-limiting, Myocardial infarction, Pulmonary edema, Pulmonary fibrosis, Renal failure, Respiratory failure, Reversible posterior leukoencephalopathy syndrome, Seizure, Thrombotic microangiopathy, Tissue necrosis, Vasculitis THERAPEUTIC USE: Antihyperlipidemic, Fibric Acid DOSE: Adult – 600 mg ORALLY twice daily 30 minutes before the morning and evening meal. Pedia – safety and efficacy not established in pediatric patients CONTRA-INDICATIONS: Concomitant gemfibrozil and cerivastatin, Pre-existing gallbladder disease, Hepatic dysfunction, including primary biliary cirrhosis, Hypersensitivity to gemfibrozil, Severe renal impairment ADVERSE EFFECTS: Liver function tests abnormal, Rhabdomyolysis, Especially when coadministered with a statin THERAPEUTIC USE: Aminoglycoside, Antibacterial, Antibiotic DOSE: Adult – Bacterial endocarditis a)(traditional dosing) 3 mg/kg/d IV in equally divided doses every 8 h or up to 5 mg/kg/d IV in 3 or 4 equally divided doses; adjust dose based on serum concentrations b)(once daily dosing, not FDA approved) 4 to 7 mg/kg/day IV once every 24 h; adjust dosage based on serum concentrations. Pedia – Bacterial sepsis of newborn a)(neonates, less than 28 weeks gestational age) 2.5 mg/kg IV every 24-36 h b)(neonates, 28-32 weeks 30 PRODUCT DESCRIPTION TRADE NAME Gentam Gentamicin / Betamethasone Garasone Glibenclamide (Glyburide) Daonil Diatab Euglucon Glibil Glynase Gliclazide Diamicron Glaze Glizide Glipizide Glucagon Glucose Amaryl Diapride Minidiab PRODUCT INFORMATION Injection 80mg/2mL gestational age) 2.5 mg/kg IV every 18 h c)(neonates, 33-42 weeks gestational age) 2.5 mg/kg IV every 12 h d)(term neonates over 1 week of Eye/Ear Drops Crystals Solution 1% Gentian Violet Glimepiride DOSAGE FORM/STRENGTH Tablet 5mg Tablet 80mg Tablet 2mg Tablet 3mg Tablet 5mg Sucrazide Injection 1unit (1mg) Powder 50grams Powder 75grams Powder 100grams Powder 450grams age, infants and children less than 5 years of age) 2.5 mg/kg IV every 8 h e)(children 5 years and older) 2-2.5 mg/kg IV every 8 h CONTRA-INDICATIONS: hypersensitivity to gentamicin/aminoglycosides ADVERSE EFFECTS: Nephrotoxicity, Neuromuscular blockade finding, Ototoxicity, Respiratory tract paralysis, Concomitant anesthesia, muscle relaxants THERAPEUTIC USE: Aminoglycoside, Antibacterial, Antibiotic DOSE: Adult – Eye infection a)ophthalmic ointment 0.3%, apply a small amount (half-inch ribbon) 2 to 3 times a day b)ophthalmic solution 0.3%, instill 1 to 2 drops into the affected eye every 4 h, up to 2 drops every hour for severe infections CONTRA-INDICATIONS: hypersensitivity to gentamicin/aminoglycosides THERAPEUTIC USE: Antifungal, Antisepsis skin cleansing procedure DOSE: Adult - Antisepsis Skin cleansing procedure, apply to affected area once or twice daily CONTRA-INDICATIONS: hypersensitivity to gentian violet, patients with porphyria, use on ulcerative lesions or open wounds THERAPEUTIC USE: 2nd Generation Sulfonylurea, Hypoglycemic DOSE: Adult – Diabetes mellitus type 2 a)(regular) initial, 2.5-5 mg ORALLY once daily b)(regular) maintenance, 1.25-20 mg ORALLY once daily or in 2 divided doses, MAX 20 mg/day c)(micronized glyburide): initial, 1.5-3 mg ORALLY once daily d)(micronized glyburide): maintenance, 0.75-12 mg ORALLY once daily or in 2 divided doses, MAX 12 mg/day. Pedia – safety and efficacy not established in pediatric patients CONTRA-INDICATIONS: diabetic ketoacidosis, hypersensitivity to glyburide ADVERSE EFFECTS: Hypoglycemia THERAPEUTIC USE: 2nd Generation Sulfonylurea, Hypoglycemic DOSE: Adult – initial doses of 40 or 80 mg orally daily with breakfast. The dose is increased in increments of 40 or 80 mg (usually weekly) until adequate glycemic control is achieved. The drug can be given once daily, although twice-daily therapy (with meals) may be indicated in some patients. Maximum doses have usually been 320 mg daily CONTRA-INDICATIONS: Diabetic ketoacidosis, Hypersensitivity to gliclazide or other sulfonylureas, Type I diabetes as sole therapy THERAPEUTIC USE: 2nd Generation Sulfonylurea, Hypoglycemic DOSE: Adult – Diabetes mellitus type 2 a)initial, 1 to 2 mg ORALLY once daily b)maintenance, 1 to 4 mg ORALLY once daily, MAX 8 mg daily. Pedia – not FDA-approved in pediatric patients.Diabetes mellitus type 2 a)initial, 1 mg ORALLY once daily b)maintenance, 1 to 8 mg ORALLY once daily, MAX 8 mg daily CONTRA-INDICATIONS: diabetic ketoacidosis, hypersensitivity to glimepiride products, pregnancy complicated by diabetes mellitus ADVERSE EFFECTS: Hypoglycemia THERAPEUTIC USE: 2nd Generation Sulfonylurea, Hypoglycemic DOSE: Adult – Diabetes mellitus type 2 a)(Glucotrol): initial, 5 mg ORALLY once daily b)(Glucotrol): maintenance, MAX 40 mg daily (if dose exceeds 15 mg, divide into at least 2 doses) c)(Glucotrol-XL): initial: 5 mg ORALLY once daily d)(Glucotrol-XL): maintenance: 5-10 mg ORALLY daily, MAX 20 mg daily. Pedia – safety and efficacy not established in pediatric patients CONTRA-INDICATIONS: diabetic ketoacidosis, hypersensitivity to glipizide ADVERSE EFFECTS: Hypoglycemia, Stevens-Johnson syndrome THERAPEUTIC USE: Beta-Adrenergic Blocker Antagonist, Diagnostic Agent, Gastric Function, Gastrointestinal Agent, Glucose Regulation, Antihypoglycemic DOSE: Adult – Hypoglycemia 1 mg (1 unit) IM,IV,SC; Radiography of gastrointestinal tract a)(IV) 0.25 to 2 mg (0.25 to 2 units) IV 1 min before procedure b)(IM) 1 to 2 mg (1 to 2 units) IM 4 to 10 min before procedure. Pedia – Hypoglycemia a)(weight <20 kg (<44 pounds)): 0.02 to 0.03 mg/kg/dose or 0.5 mg/dose IM,IV,SC; MAX 1 mg/dose b)(weight >20 kg (>44 pounds): 1 mg (1 unit) IV, IM, SC CONTRA-INDICATIONS: hypersensitivity to glucagon, known pheochromocytoma THERAPEUTIC USE: Diagnostic Agent, Nutritive Agent, Osmotherapy Agent, Parenteral Solution DOSE: Adult – Glucose tolerance test a)Diabetes mellitus (diagnosis): 75 g anhydrous glucose (dextrose) dissolved in water ORALLY, as one-time dose for oral glucose tolerance test b)Gestational diabetes mellitus (diagnosis), one-step approach: 100 g anhydrous glucose (dextrose) dissolved in water ORALLY, as one-time dose for oral glucose tolerance test c)Gestational diabetes mellitus (diagnosis), two-step approach: 50 g anhydrous glucose (dextrose) dissolved in water ORALLY as one-time dose for glucose challenge test, then perform the 100g oral glucose tolerance test on those who exceeded the threshold value on the glucose challenge test CONTRA-INDICATIONS: a)Do not use concentrated solutions of dextrose in patients with: anuria, diabetic coma and hyperglycemia, intracranial or intraspinal hemorrhage, delirium tremens in dehydrated patients, glucose-galactose malabsorption syndrome b)Allergy to corn or corn products 31 PRODUCT DESCRIPTION TRADE NAME DOSAGE FORM/STRENGTH Glycerin Laxolyne Liquid Supp. Adult Supp. Pedia Glyceryl Trinitrate Deponit NT Minitran Nitroderm TTS Tridil Inj. 50mg/10mL Inj. 50mg/50mL Patch 5mg Patch 10mg Glycopyrronium Bromide Inj. 0.2mg/mL Goserelin Acetate Zoladex Depot Inj. 3.6mg Granisetron HCl Kytril Inj. 1mg/mL Tablet 1mg Guaphan Guaifenesin Resyl Syrup 100mg/5mL Robitussin Haemophilus Influenza Type B Conjugated Vaccine Hibtiter Inj. 10mcg/0.5mL PRODUCT INFORMATION THERAPEUTIC USE: Antiglaucoma, Cardiovascular Agent, Diuretic, Osmotic, Gastrointestinal Agent, Laxative, Hyperosmotic, Laxative, Stool Softener, Lubricant, Ocular Protectant, Dental, Protectant, Dermatological DOSE: Adult – is 3 grams suppositories should be retained for 15 minutes; liquid glycerin is administered rectally, gently insert stem with steady pressure with the tip pointing toward the navel and squeeze the unit until almost all the liquid has been delivered; a small amount of liquid will remain. Pedia – younger than 6 years of age of glycerin suppositories to promote fecal evacuation is 1 to 1.5 grams; suppositories should be retained for 15 minutes CONTRA-INDICATIONS: Hypersensitivity to any component in the preparation, Well-established anuria, Severe dehydration, Frank or impending acute pulmonary edema, Severe cardiac decompensation THERAPEUTIC USE: Antianginal, Coronary Vasodilator, Nitrate DOSE: Adult – Pulmonary edema a)initial, 5 to 10 mcg/min IV, titrate in increments of 5 mcg/min every 3 to 5 min to total dose of 100 to 200 mcg/min until desired hemodynamic effect obtained; systolic arterial pressure should be kept in the range of 90 to 100 mmHg b)SL tablet; 0.3 to 0.4 mg every 5 min x 3. Pedia – safety and efficacy not established in children. Pulmonary edema, initial, 0.5 to 20 mcg/kg/min IV; MAX, 60 mcg/kg/min CONTRA-INDICATIONS: anemia, severe, concurrent use of phosphodiesterase inhibitors such as sildenafil or vardenafil (increased hypotensive effect), constrictive pericarditis, early myocardial infarction, hypersensitivity to adhesives, hypersensitivity to organic nitrates, increased intracranial pressure, pericardial tamponade, restrictive cardiomyopathy, symptomatic hypotension ADVERSE EFFECTS: Blood coagulation disorder with prolonged bleeding time, Methemoglobinemia, Rebound hypertension, Uncommon, Scaling eczema, Syncope, Thrombocytopenia, Unstable angina THERAPEUTIC USE: Antimuscarinic, Cholinergic Antagonist, Gastrointestinal Agent DOSE: Adult – Cardiac dysrhythmia, Vagal reflex associated, surgically-induced or drug-induced – Surgical procedure, 0.1 mg IV as a single dose, repeat every 2 to 3 min as needed. Pedia - a)glycopyrrolate injection contains benzyl alcohol; do not use in patients less than 1 month of age b)safety and effectiveness in pediatric patients below the age of 16 years have not been established CONTRA-INDICATIONS: gastrointestinal obstruction, glaucoma, hypersensitivity to glycopyrrolates, myasthenia gravis, newborns less than 1 month of age, obstructive uropathy, paralytic ileus or intestinal atony, severe ulcerative colitis or toxic megacolon, unstable cardiovascular status in acute hemorrhage ADVERSE EFFECTS: Allergic reaction, acute, Anaphylaxis, Cardiac arrest, Cardiac dysrhythmia, Malignant hyperthermia, Seizure, Tachyarrhythmia THERAPEUTIC USE: Antineoplastic Agent, Luteinizing Hormone Releasing Hormone Agonist DOSE: Adult – Breast cancer, For palliation of advanced disease in pre- and peri-menopausal women, 3.6 mg SubQ every 28 days for longterm therapy CONTRA-INDICATIONS: hypersensitivity to goserelin products, leutenizing hormone-releasing hormone (LHRH) or LHRH analogues, 10.8 mg depot dose in women ADVERSE EFFECTS: Deep venous thrombosis THERAPEUTIC USE: Antiemetic, Serotonin Receptor Antagonist, 5-HT3 DOSE: Adult – Chemo-induced nausea and vomiting; Prophylaxis a)2mg orally 1hr before chemo or 1mg orally 1 hr before and 1mg 12 hr after chemo b)10 mcg/kg IV 30 min before chemo. Pedia - 2 yrs or older, 10 to 40 mcg/kg/dose IV 30 min before chemo. CONTRA-INDICATIONS: hypersensitivity to granisetron or any of its components THERAPEUTIC USE: Expectorant DOSE: Adult – Sputum abnormal – amount a)liquid/syrup/immediate-release, 200 to 400 mg orally every 4 h; max.dose, 2400 mg/day b)sustained-release, 600 to 1200 mg orally every 12 h; max.dose, 2400 mg/day. Pedia – a)(12 y and older) liquid/syrup/immediate-release, 200 to 400 mg orally every 4 h; max.dose, 2400 mg/day b)(12 y and older) sustained-release, 600 to 1200 mg orally every 12 h; max.dose, 2400 mg/day c)(6 to 12 y) liquid/syrup, 100 to 200 mg orally every 4 h; max.dose, 1200 mg/day d)(2 to 6 y) liquid/syrup, 50 to 100 mg orally every 4 h; max.dose, 600 mg/day e)(under 2 y) dose should be individualized, common dosage is 25 to -50 mg orally every 4 h; max. dose, 300 mg/day CONTRA-INDICATIONS: hypersensitivity to guaifenesin products THERAPEUTIC USE: For the prevention of invasive Haemophilus influenzae tybe infections (meningitis, septicaemia, cellulitis, arthritis, epiglottitis) in infants from 2months of age. DOSE: Pedia – Before 6 months of age, 3 successive 0.5mL doses at one or two month intervals followed by a booster injection at 18months of age; 6 – 12 months, two 0.5mL doses at a 1month interval followed by a booster injection (0.5mL) at 18months of age; From 1 to 5 years, a single 0.5mL dose. CONTRA-INDICATIONS: Hypersensitivity of one of the ingredients of the vaccine, particularly tetanus protein or allergy appearing after a previous injection of conjugated Haemophilus influenzae type b vaccine 32 PRODUCT DESCRIPTION Haloperidol TRADE NAME Haldol Serenace DOSAGE FORM/STRENGTH Drops 2mg/mL Inj. 5mg/mL Tablet 1.5mg Tablet 5mg Halothane Anesthane Inhalation Heparenoid Hirudoid Lasonil Prelloran Ointment 0.8% Inj. 1000iu/mL Inj. 5000iu/mL Inj. 25,000iu/mL Heparin Sodium Hepatitis B Immunoglobulin Hepatect Injection Hepatitis B Vaccine Engerix-B Injection Adult Injection Pedia Human Antitetanus Immunoglobulin Tetagam P Injection 250iu/mL Pregnyl Injection PRODUCT INFORMATION THERAPEUTIC USE: Antipsychotic, Butyrophenone, Dopamine Antagonist DOSE: Adult – Schizophrenia a)2 to 5 mg IM, may repeat every 4 to 8 h depending on patient response; increase to every 1 h if needed b)0.5 to 2 mg (moderate symptoms) or 3 to 5 mg (severe symptoms) orally 2 to 3 times daily. Pedia – Not FDA approved in children less than 3 years of age. Schizophrenia a)3 to 12 y (weight range 15 to 40 kg), 0.05 mg/kg/day orally in 2 to 3 divided doses, may increase by 0.5 mg/day at 5 to 7 day intervals to therapeutic effect; Max. daily dose 0.15 mg/kg/day b)12 y and older, 0.5 to 2 mg (moderate symptoms) or 3 to 5 mg (severe symptoms) orally 2 to 3 times daily CONTRA-INDICATIONS: comatose states, hypersensitivity to haloperidol, Parkinson's disease, toxic central nervous system depression, severe ADVERSE EFFECTS: Agranulocytosis, Dead – sudden death, Neuroleptic malignant syndrome, Paralytic ileus, Priapism, Prolonged QT interval, Seizure, Tardive dyskinesia, Torsades de pointes THERAPEUTIC USE: Haloalkane, Volatile Liquid DOSE: Adult – Inhalation route, For induction of ANESTHESIA, halothane doses vary from patient to patient. Usual concentrations have been 0.5% to 3% in oxygen or nitrous oxide/oxygen. Pedia – For induction of anesthesia, a concentration of 0.5% to 2.5% in nitrous oxide/oxygen has been used in children CONTRA-INDICATIONS: Hypersensitivity to halothane or other halogenated volatile anesthetics, Obstetrical anesthesia unless uterine relaxation is required, Patients with known or suspected susceptibility to malignant hyperthermia, Patients who have developed jaundice or other signs of acute hepatic damage after previous exposure to halothane THERAPEUTIC USE: Bruising, Injuries to unbroken skin (soft tissue injuries), Sprains ADVERSE EFFECTS: Flushing of the skin due to widening of the small blood vessels, Allergy to one or more of the ingredients (hypersensitivity) PRECAUTIONS: Broken skin or open wounds, Children under 12 years of age, Infected wounds or ulcers, Large areas of skin, Lining of the body's cavities (mucous membranes) THERAPEUTIC USE: Anticoagulant, Heparin (class) DOSE: Adult – Acute coronary syndrome, 60 to 70 units/kg IV bolus (maximum 5000 units) then 12 to 15 units/kg/hr (maximum 1000 units/hr). Pedia – use of 100 unit/mL heparin flush not recommended for use in neonates; do not use solutions preserved with benzyl alcohol in neonates. Anticoagulant therapy – Collection of blood specimen for laboratory,70 to 150 units/10 to 20 mL sample of whole blood CONTRA-INDICATIONS: active bleeding, uncontrollable; except when due to disseminated intravascular coagulation, instances in which blood coagulation tests cannot be performed at necessary intervals, severe thrombocytopenia ADVERSE EFFECTS: Anaphylaxis, Hemorrhage, Heparin-induced thrombocytopenia with thrombosis, Delayed, Immune hypersensitivity reaction, Increased liver aminotransferase level, Osteoporosis, With long-term, high-dose administration THERAPEUTIC USE: Vaccine DOSE: Adult – Hepatitis B, Post-exposure; Prophylaxis, household exposure, blood exposure (sharing toothbrushes, razors); 0.06 mL/kg IM as a single dose within 14 days of exposure, plus hepatitis B vaccine series. Pedia – Hepatitis B, Post-exposure; Prophylaxis, household exposure in infants less than 12 months of age, acute hepatitis B infection in mother or caregiver – 0.5 mL IM as a single dose plus hepatitis B vaccine CONTRA-INDICATIONS: anaphylactic or severe systematic reactions to parenteral human globulin, IgA deficiency; increased risk of anaphylactoid reaction THERAPEUTIC USE: Vaccine DOSE: Adult – Prophylaxis, 10 mcg (1mL) IM or 20 mcg (1 mL) IM x 3 doses at 0, 1, and 6 months. Pedia – Prophylaxis a)(0-19 yrs of age) Recombivax HB®, pediatric/adolescent formulation; 5 mcg (0.5 mL) IM x 3 doses at 0, 1, and 6 months b)(11-15 yrs of age) Recombivax HB®, adult formulation; 10 mcg (1 mL) IM x 2 doses at 0, and 4-6 months c)(0-19 yrs of age) Engerix-B®; 10 mcg (0.5 mL) IM x 3 doses at 0, 1, and 6 months CONTRA-INDICATIONS: hypersensitivity to any hepatitis-containing vaccine, hypersensitivity to yeast or any component of the vaccine ADVERSE EFFECTS: Anaphylaxis, Pancytopenia THERAPEUTIC USE: Prophylaxis in persons with recent injuries and whose prior vaccination regimen was incomplete or is unknown. Therapy of clinically manifest tetanus. DOSE: Adult & Pedia – prophylaxis of tetanus, 250 IU and 0.5mL of an adsorbed tetanus vaccine or an adequate tetanus diphtheria combination vaccine. The dose can be raised to 500 IU (simultaneously with tetanus vaccination) in other cases. CONTRA-INDICATIONS: Hypersensitivity to products containing homologous immunoglobulins, particularly in patients with IgA deficiency and concurrent presence of antibodies to IgA. THERAPEUTIC USE: Diagnostic Agent, Endocrine-Metabolic Agent, Gonadotropin DOSE: Adult – Cryptorchidism, 4000 units IM 3 times weekly for 3 weeks; OR 5000 units every second day for 4 doses; OR 15 injections of 33 PRODUCT DESCRIPTION TRADE NAME DOSAGE FORM/STRENGTH Human Chorionic Gonadotrophin Hyaluronidase Hylase Injection 1500iu Hydralazine Apresoline Inj. 20mg/mL Syrup 2mg/mL Tablet 25mg Hydrochlorothiazide Esidrex Syrup 5mg/mL Tablet 25mg Hydrocortisone Alfacort Cortiderm Hydrocortone Hydrosone Hydrocortisone Na Succinate Solu-Cortef Hydrogen Peroxide Cream 1% Syrup 2.5mg/mL Tablet 10mg Tablet 20mg Inj. 100mg/2mL Injection 250mg Solution 3% Solution 6% PRODUCT INFORMATION 500 to 1000 units over a period of 6 wks; OR 500 units 3 times weekly for 4 to 6 wks repeated 1 month later using 1000 units dose if ineffective; therapy is usually started between 4 and 9 years of age. Pedia – Cryptorchidism (4 y and older) 4000 units IM 3 times weekly for 3 weeks; OR 5000 units every second day for 4 doses; OR 15 injections of 500 to 1000 units over a period of 6 wks; OR 500 units 3 times weekly for 4 to 6 wks repeated 1 month later using 1000 units dose if ineffective; therapy is usually started between 4 and 9 years of age CONTRA-INDICATIONS: precocious puberty, prostate cancer or other androgen-dependent cancer, hypersensitivity to chorionic gonadotropin THERAPEUTIC USE: Dermatological Agent, Enzyme, Tissue Permeability Modifier DOSE: Adult – Hypersensitivity test: Intradermal injection of 0.02 mL (3 units) of a 150-unit/mL solution; positive reaction shows wheal and pseudopods and itching within 5 min, lasting for 20-30 min; typical dose, add 150 units (range 50 to 300 units) to the injection solution. Pedia - typical dose, add 150 units (range 50 to 300 units) to the injection solution; (children less than 3 yrs) MAX volume of a single clysis not to exceed 200 mL; (premature/very young infants) MAX clysis not to exceed 25 mL/kg daily; rate of infusion not to exceed 2 mL/min CONTRA-INDICATIONS: Hypersensitivity to hyaluronidase products ADVERSE EFFECTS: Anaphylaxis THERAPEUTIC USE: Antihypertensive, Peripheral Vasodilator, Peripheral Vasodilator DOSE: Adult – Essential hypertension, initial, 10 mg ORALLY four times daily for the first 2 to 4 days; increase to 25 mg ORALLY four times daily for remainder of first wk; second and subsequent wk, increase to 50 mg ORALLY four times daily; titrate to lowest effective maintenance dose; MAX dose 300 mg/day; emergency therapy: 20 to 40 mg/dose IM/IV bolus as needed. Pedia – Essential hypertension, 0.75 mg/kg/day ORALLY in 4 divided doses; increase dose gradually over 3 to 4 weeks, MAX dose 7.5 mg/kg or 200 mg daily; emergency therapy: 1.7 to 3.5 mg/kg/day IM/IV bolus in 4 to 6 divided doses CONTRA-INDICATIONS: dissecting aortic aneurysm, hypersensitivity to hydralazine ADVERSE EFFECTS: Agranulocytosis, Hepatotoxicity, Leukopenia, Lupus pneumonia, Systemic lupus erythematosus THERAPEUTIC USE: Cardiovascular Agent, Diuretic, Thiazide DOSE: Adult – Hypertension a)initial, 12.5 to 25 mg ORALLY once daily b)titration, allow 2-3 wk to achieve optimum antihypertensive effect c)titrate to maintenance, may increase to 50-100 mg ORALLY daily in single or divided doses. Pedia – Hypertension a)1 to 2 mg/kg ORALLY daily in single or two divided doses; infants less than 6 months old, may require doses up to 3 mg/kg ORALLY daily in two divided doses b)infants up to 2 yrs old: MAX dose, not to exceed 37.5 mg ORALLY daily c)children 2-12 yrs old: MAX dose, not to exceed 100 mg ORALLY daily CONTRA-INDICATIONS: anuria, hypersensitivity to hydrochlorothiazide or sulfonamides ADVERSE EFFECTS: Angle-closure glaucoma, Cardiac dysrhythmia, Disorder of hematopoietic structure, Hepatotoxicity, Pancreatitis, Pulmonary edema, Scaling eczema, Stevens-Johnson syndrome, Systemic lupus erythematosus, Toxic epidermal necrolysis THERAPEUTIC USE: Adrenal Glucocorticoid, Anti-Inflammatory, Corticosteroid, Endocrine-Metabolic Agent, Gastrointestinal Agent, Hemorrhoidal Anti-Inflammatory, Immune Suppressant, Otic Agent DOSE: Adult – Allergic disorder, 20 to 240 mg per day ORALLY; Disorder of skin, ORAL; 20 to 240 mg per day, TOPICAL; apply to affected area as a thin film 2 to 4 times daily. Pedia – Disorder of skin TOPICAL: apply to affected area as a thin film 2 to 4 times daily; Nephrotic syndrome, Idiopathic or due to lupus erythematosus; 240 mg/m(2) ORALLY daily; MAX 320 mg/day; divided 3 times/day for 4 wk, then 160 mg/m(2) every other day for 4 wk CONTRA-INDICATIONS: hypersensitivity to hydrocortisone or any of its components, live or live attenuated vaccines, systemic fungal infections ADVERSE EFFECTS: Adrenal insufficiency, Cataract, Cushing's syndrome, Disorder of fluid &/or electrolyte, Drug-induced adrenocortical insufficiency, HPA, Glaucoma, Hyperglycemia, Pulmonary tuberculosis THERAPEUTIC USE: Adrenal Glucocorticoid, Anti-Inflammatory, Corticosteroid, Endocrine-Metabolic Agent, Gastrointestinal Agent, Hemorrhoidal Anti-Inflammatory, Immune Suppressant, Otic Agent DOSE: Adult – Adrenal insufficiency – Surgical procedure, 100 to 500 mg IM or IV over 30 seconds to 10 min every 2 to 6 hours. CONTRA-INDICATIONS: hypersensitivity to hydrocortisone or any of its components, live or live attenuated vaccines, systemic fungal infections ADVERSE EFFECTS: Adrenal insufficiency, Cataract, Cushing's syndrome, Disorder of fluid &/or electrolyte, Drug-induced adrenocortical insufficiency, HPA, Glaucoma, THERAPEUTIC USE: Antiseptic, Disinfectant DOSE: Adult – Oral hygiene finding, Cleansing minor wounds or minor gum inflammation a)rinse in mouth with 10 mL for approximately 1 min, then spit out; use up to 4 times daily (after meals and at bedtime) b)gel, apply topically several drops to affected area of mouth; remain in place at least one minute then spit out; use up to 4 times daily (after meals and at bedtime). Pedia – Oral hygiene finding, Cleansing minor wounds or minor gum inflammation a)(2 y or older) rinse in mouth with 10 mL for approximately 1 min, then spit out; use up to 4 times daily 34 PRODUCT DESCRIPTION TRADE NAME Injection 1mg/mL Hydromorphone Hydroxychloroquine Sulphate Hydroxyurea DOSAGE FORM/STRENGTH Hydroquine Tablet 200mg Plaquenil Hydrea Capsule 500mg Buscopan Hyoscine-N-Butyl Bromide Scopinal Inj. 20mg/mL Tablet 10mg Spasmopan Hypromellose Ibuprofen Artificial Tears Eye Drops 0.3% Tears Naturale Advil Brufen Dolgit Profinal Sabopen Syrup 100mg/5mL Tablet 400mg PRODUCT INFORMATION (after meals and at bedtime) b)(2 y or older) gel, apply topically several drops to affected area of mouth; remain in place at least one minute then spit out; use up to 4 times daily (after meals and at bedtime) CONTRA-INDICATIONS: Injection or instillation into closed body cavities from which the released oxygen has no free exit THERAPEUTIC USE: Analgesic, Anesthetic Adjunct, Opioid DOSE: Adult - Chronic pain (Moderate to Severe) 3 to 4 mg IM/SC every 3 to 4 h; opioid tolerant patients, 1 to 14 mg IM/SubQ. Pedia - Pain, acute (Moderate to Severe) adolescents, initial (opiate-naive patients) 0.2 to 0.6 mg IV (slow) every 2 to 3 h as needed; usual, 1 to 2 mg/dose IM/IV/SubQ every 4 to 6 h; children, 0.015 mg/kg/dose IV every 4 to 6 h. CONTRA-INDICATIONS: bronchial asthma, acute or severe, depressed ventilatory function; such as COPD, cor pulmonale, emphysema, kyphoscoliosis, hypersensitivity to hydromorphone, intracranial lesion associated with increased intracranial pressure, obstretical analgesia, paralytic ileus, known or suspected, patients not already receiving large amounts of parenteral opioids, respiratory depression in the absence of resuscitative equipment, status asthmaticus, use of extended-release capsules on an as needed basis ADVERSE EFFECTS: Apnea, Confusion, Disorder of cardiovascular system, Hypotension, Myoclonus, Respiratory depression, Seizure THERAPEUTIC USE: Aminoquinoline, Antimalarial, Antirheumatic DOSE: Adult – Malaria a)suppression: 400 mg (310 mg base) ORALLY once weekly on the same day; begin 2 weeks prior to entering an endemic area and continue for 8 weeks after leaving the endemic area b)loading dose (if unable to start suppressive therapy 2 weeks prior): 800 mg (620 mg base) ORALLY initially, followed by normal suppressive therapy c)acute attack: 800 mg (620 mg base) ORALLY initially, then 400 mg (310 mg base) in 6 to 8 hours and 400 mg once daily for 2 days OR a single dose of 800 mg ORALLY. Pedia – Malaria a)suppression: 5 mg base/kg ORALLY once weekly on the same day; begin 2 weeks prior to entering an endemic area and continue for 8 weeks after leaving the endemic area b)loading dose (if unable to start suppressive therapy 2 weeks prior): 10 mg base/kg ORALLY in two divided doses 6 hours apart, followed by normal suppressive therapy c)acute attack: 10 mg base/kg (MAX 620 mg base) ORALLY (first dose), followed by 5 mg base/kg (MAX 310 mg base) ORALLY in 6 hours (second dose), then 18 hours after second dose (third dose), then 24 hours after third dose (fourth dose) CONTRA-INDICATIONS: hypersensitivity to 4-aminoquinoline compound, long-term use in children, retinal or visual field changes from prior 4-aminoquinoline compound ADVERSE EFFECTS: Agranulocytosis, Ototoxicity, Retinopathy, Torsades de pointes THERAPEUTIC USE: Antimetabolite, Antineoplastic Agent DOSE: Adult – Chronic myeloid leukemia, Resistant, Continuous therapy, 20 to 30 mg/kg ORALLY given as a single daily dose; continue administration for a minimum of 6 weeks to assess effectiveness and continue indefinitely if effective. Pedia - safety and efficacy not established in pediatric patients. CONTRA-INDICATIONS: hypersensitivity to hydroxyurea products, severe bone marrow depression ADVERSE EFFECTS: Gangrenous disorder, Genetic mutation, Long-term use, Secondary leukemia, Long-term use, Skin ulcer THERAPEUTIC USE: Anesthetic Adjunct, Antiemetic, Antimuscarinic, Antivertigo, Gastrointestinal Agent, Mydriatic-Cycloplegic DOSE: Adult – Increased intestinal motility, 0.4 to 0.8 mg; Mania 0.6 mg IM/IV/SC. Pedia – not FDA approved in pediatric patients. Preoperative sedation a)Injection: (age 6 months to 3 years) 0.1-0.15 mg IM/IV/SC b)Injection: (age 3 to 6 years) 0.2-0.3mg IMIV/SC; Vomiting – Injection: 6 mcg/kg/dose SC CONTRA-INDICATIONS: chronic lung disease; repeated administration may increase the risk of adverse events, hepatic impairment, hypersensitivity/idiosyncrasy to scopolamine hydrobromide, other belladonna alkaloids or anticholinergic drugs, or barbiturates, narrow-angle (angle-closure) glaucoma, prostatic hypertrophy, pyloric obstruction renal impairment ADVERSE EFFECTS: Confusion, Systemic absorption, Dry skin, Systemic absorption Hallucinations, Somnolence, Systemic, Tachyarrhythmia Xerostomia, Systemic absorption of ophthalmic formulation THERAPEUTIC USE: Diagnostic Agent, Lubricant, Ocular, Surgical Aid, Ocular DOSE: Adult – Gonioscopy; Diagnosis, 2.5% OPHTHALMIC solution used as needed to fill the gonioscopic prism; Operative procedure on anterior chamber of eye – instill a 2% OPHTHALMIC solution into the anterior chamber, as needed, using a 20-gauge or larger cannula; Xerophthalmia – instill 1 drop 0.3% to 1% OPHTHALMIC solution into affected eye(s) 3 to 4 times daily, as needed. Pedia – Xerophthalmia (adolescents) instill 1 drop 0.3% to 1% OPHTHALMIC solution into affected eye(s) 3 to 4 times daily, as needed CONTRA-INDICATIONS: hypersensitivity to hypromellose THERAPEUTIC USE: Analgesic, Antimigraine, Antirheumatic, Central Nervous System Agent, Musculoskeletal Agent, NSAID, Propionic Acid (class) DOSE: Adult – Pain over-the-counter dosing, 200 to 400 mg ORALLY every 4 to 6 hours as needed, MAX 1200 mg/day; do not take longer than 10 days unless directed by a physician. Pedia – Pain over-the-counter dosing, 6 months-12 years old, 5 to 10 mg/kg ORALLY every 6 to 8 hours as needed, MAX 4 doses/day; 12 years and older, 200 to 400 mg ORALLY every 4 to 6 hours as needed, MAX 1200 mg/day; do not give longer than 10 days unless directed by a physician 35 PRODUCT DESCRIPTION TRADE NAME DOSAGE FORM/STRENGTH Sapofen Ointment 10% Powder Ichthammol Idarubicin Zavedos Injection 10mg/10mL Ifosfamide Holoxan Injection 1gram Injection 2grams Imipenem-Cilastatin Tienam Injection 500mg Imipramine HCl Tofranil Tablet 10mg Tablet 25mg PRODUCT INFORMATION CONTRA-INDICATIONS: treatment of peri-operative pain in setting of coronary artery bypass graft, bleeding, especially those with active intracranial hemorrhage or gastrointestinal bleeding, coagulation defects, congenital heart disease in whom patency of the ductus arteriosus is necessary for satisfactory pulmonary or systemic blood flow, hypersensitivity to ibuprofen, infection that is untreated, proven or suspected, necrotizing enterocolitis, present or suspected, patients who have experienced asthma, urticaria, or allergic-type reactions after taking aspirin or other nonsteroidal anti-inflammatory agents; severe, even fatal, anaphylactic-like reactions have been reported renal function impairment, significant, thrombocytopenia ADVERSE EFFECTS: Acute renal failure, Agranulocytosis, Amblyopia, Anaphylactoid reaction, Anemia, Aseptic meningitis , Cerebrovascular accident, Confusion, Congestive heart failure, Depression, Erythema multiforme, Gastrointestinal hemorrhage, perforation & ulcer, Hearing loss, Hematuria, Hemolytic anemia, Hepatitis, Hypertension, Inflammatory disorder of digestive tract, Jaundice, Melena, Myocardial infarction, Neutropenia, Pancreatitis, Renal azotemia, Scaling eczema, Stevens-Johnson syndrome, Thrombocytopenia, Thrombotic tendency observations, Toxic epidermal necrolysis THERAPEUTIC USE: An irritant & local antibacterial agent. It is used alone or in combination with other antiseptics for the treatment of skin disorders such as erysipelas, psoariasis and lupus erythematosus, and to promote healing in chronic inflammations. DOSE: Topical to the skin as a 10% ointment. THERAPEUTIC USE: Anthracycline, Antineoplastic Agent DOSE: Adult – Acute myeloid leukemia, In combination with other approved antileukemic agents, induction, 12 mg/m(2) IV over 10 to 15 min daily for 3 days in combination with cytarabine; cytarabine can be dosed as 100 mg/m(2) every day by continuous infusion for 7 days or as 25 mg/m(2) IV bolus followed by 200 mg/m(2) every day by continuous infusion for 5 days. Pedia – Safety and effectiveness in children have not been established. Acute lymphoid leukemia 8 to 10 mg/m(2) IV daily for 3 days has been used CONTRA-INDICATIONS: hypersensitivity to idarubicin or other anthracyclines, marked myelosuppression induced by previous treatment with other antitumor agents or by radiotherapy, previous treatment with complete cumulative doses of DOXORUBICIN or daunorubicin, idarubicin, or other anthracyclines ADVERSE EFFECTS: Cardiac dysrhythmia, Chest pain, Congestive heart failure, Hepatotoxicity, Infectious disease, Inflammatory disease of mucous membrane, Myelosuppression, Myocardial infarction, Nephrotoxicity THERAPEUTIC USE: Alkylating Agent, Antineoplastic Agent, Nitrogen Mustard DOSE: Adult – Testicular cancer, Germ cell; third-line, in combination with other antineoplastic agents, 1.2 g/m(2) IV daily for 5 days repeated every 3 weeks; use with a prophylactic agent for hemorrhagic cystitis. Pedia – Safety and effectiveness in children have not been established CONTRA-INDICATIONS: hypersensitivity to ifosfamide or mesna products, severe bone marrow depression ADVERSE EFFECTS: Disorder of urinary tract, Metabolic acidosis, Myelosuppression, Nephrotoxicity, Neurotoxicity THERAPEUTIC USE: Antibiotic DOSE: Adult – Bacterial endocarditis a)mild infection: (fully susceptible organisms) 250 mg IV every 6 h; (moderately susceptible organisms including P. aeruginosa) 500 mg IV every 6 h; maximum 50 mg/kg/day b)moderate infection (fully susceptible organisms) 500 mg IV every 6 to 8 h; (moderately susceptible organisms including P. aeruginosa) 500 mg IV every 6 h or 1 g IV every 8 h; maximum 50 mg/kg/day c)severe infection (fully susceptible organisms) 500 mg IV every 6 h; (moderately susceptible organisms including P. aeruginosa) 1 g IV every 8 h or 1 g IV every 6 h; maximum 50 mg/kg/day. Pedia – Safety and efficacy of the intramuscular product in children under 12 years of age have not been established. Bacterial endocarditis a)(less than 1 week of age) 25 mg/kg IV every 12 h; maximum 2 g/day for fully susceptible organisms, maximum 4 g/day for moderately susceptible organisms including P. aeruginosa b)(1 to 4 weeks of age) 25 mg/kg IV every 8 h; maximum 2 g/day for fully susceptible organisms, maximum 4 g/day for moderately susceptible organisms including P. aeruginosa c)(4 weeks to 3 months of age) 25 mg/kg IV every 6 h; maximum 2 g/day for fully susceptible organisms, maximum 4 g/day for moderately susceptible organisms including P. aeruginosa d)(3 months of age and older) 15 to 25 mg/kg IV every 6 h; maximum 2 g/day for fully susceptible organisms, maximum 4 g/day for moderately susceptible organisms including P. aeruginosa CONTRA-INDICATIONS: hypersensitivity to imipenem or cilastatin, or any component of the product, hypersensitivity to local anesthetics of the amide type and in patients with severe shock or heart block; IM product diluted with lidocaine ADVERSE EFFECTS: Anaphylaxis, Seizure THERAPEUTIC USE: Antidepressant Tricyclic, Urinary Enuresis Agent DOSE: Adult – Depression a)(hospitalized patients) 100 mg ORALLY per day in divided doses; may increase up to a MAX of 300 mg/day b)(outpatients) 75 mg ORALLY per day; may increase up to a MAX of 200 mg/day; usual maintenance dose, 50 to 150 mg/day. Pedia – safety and effectiveness in children with nocturnal enuresis below the age of 6 years have not been established; effectiveness in pediatric patients for any other condition has not been established. Nocturnal enuresis a)(children 6 to 12 y) initial, 25 mg ORALLY 1 h before bedtime, may increase in 25 mg increments to MAX dose of 50 mg/d or 2.5 mg/kg/d b)(children over 12 y) initial, 25 mg ORALLY 1 h before bedtime, may increase in 25 mg increments to MAX dose of 75 mg/d or 2.5 mg/kg/d 36 PRODUCT DESCRIPTION Immunoglobulin Varicella Zoster TRADE NAME Varitect DOSAGE FORM/STRENGTH Injection Injection 2.5grams Injection 5grams Immunoglobulin Human Indapamide Natrilix SR Tablet 1.5mg Tablet 2.5mg Indomethacin Indocid Capsule 25mg Injection 1mg Supp. 100mg Insulin Isophane (NPH) Insulatard NPH Inj. 100u/mL Insulin Soluble (Regular) Interferon Alfa-2b Human Actrapid Humulin Intron – A Inj. 100u/mL Injection 3miu PRODUCT INFORMATION CONTRA-INDICATIONS: coadministration with a MAOI or use within 14 days of discontinuing a MAOI; may cause serious reactions, hypersensitivity to dibenzazepines; risk of cross-sensitivity reactions, hypersensitivity to imipramine hydrochloride myocardial infarction, during the acute recovery period ADVERSE EFFECTS: Agranulocytosis, Atrioventricular conduction pattern – finding, Cardiac dysrhythmia, Cerebrovascular accident, Decreased liver function, Depression, worsening, Heart block, Hypertension, Jaundice, Myocardial infarction, Orthostatic hypotension, Palpitations, Psychotic disorder, Seizure, Suicidal thoughts, Suicide, Syncope THERAPEUTIC USE: Immune Serum DOSE: Adult – Varicella; Prophylaxis, 125 international units/10 kg IM or IV; MAX dose, 625 international units and MIN dose, 125 international units. Pedia – Prophylaxis, 125 international units/10 kg IM or IV; MAX dose, 625 international units and MIN dose, 125 international units CONTRA-INDICATIONS: IgA deficiency; potential to develop IgA antibodies and subsequent anaphylactoid reaction, history of anaphylactic or other severe systemic reaction to immune globulins, hypersensitivity to any ingredient in the formulation or component of the container, known immunity to varicella zoster virus ADVERSE EFFECTS: Anaphylaxis THERAPEUTIC USE: Immune Serum DOSE: Adult & Pedia – FDA-approved indications vary by specific product. Bacterial infectious disease; Prophylaxis – B-cell chronic lymphocytic leukemia 400 mg/kg IV every 3 to 4 weeks. CONTRA-INDICATIONS: history of severe systemic allergic reaction to human immunoglobulin products, selective IgA deficiency, severe thrombocytopenia or any coagulation disorder which would contraindicate intramuscular injections ADVERSE EFFECTS: Acute renal failure, Acute respiratory distress syndrome, Anaphylaxis, Aseptic meningitis, Bullous dermatosis, Erythema multiforme, Hemolytic anemia, Hepatitis, Hypokalemic nephropathy, Myocardial infarction, Pulmonary edema, Renal tubular disorder, Proximal, Stevens-Johnson syndrome, Thrombotic disorder, Tubular necrosis, acute THERAPEUTIC USE: Cardiovascular Agent, Diuretic, Thiazide Related DOSE: Adult – Congestive heart failure – Edema, initial, 2.5 mg ORALLY once daily in the morning; may increase to 5 mg ORALLY once daily after 1 wk; Hypertension initial, 1.25 mg ORALLY once daily in the morning; titration, allow 4 wk to achieve optimum antihypertensive effect; maintenance, 2.5 to 5 mg ORALLY once daily. Pedia – safety and effectiveness not established in children CONTRA-INDICATIONS: anuria, hypersensitivity to indapamide or sulfonamides ADVERSE EFFECTS: Cardiac dysrhythmia, Stevens-Johnson syndrome, Toxic epidermal necrolysis THERAPEUTIC USE: Acetic Acid (class), Analgesic, Antimigraine, Antipyretic, Antirheumatic, Central Nervous System Agent, Musculoskeletal Agent, NSAID DOSE: Adult – maximum recommended oral or rectal dose is 200 mg/day. Pedia – Patent ductus arteriosus a)less than 48 hours old, 0.2 mg/kg IV followed by 0.1 mg/kg IV every 12 to 24 hours for 2 doses b)2 to 7 days old, 0.2 mg/kg IV every 12 to 24 hours for 3 doses c)over 7 days old, 0.2 mg/kg IV followed by 0.25 mg/kg every 12 to 24 hours for 2 doses d)if the ductus arteriosus re-opens, a second course of 1 to 3 doses may be given CONTRA-INDICATIONS: treatment of peri-operative pain in setting of coronary artery bypass graft, history of recent rectal bleeding or proctitis, hypersensitivity to indomethacin, neonates with active bleeding, infection, necrotizing enterocolitis, severe renal failure, thrombocytopenia, or coagulation defects, patients who have experienced asthma, urticaria, or allergic-type reactions after taking aspirin or other nonsteroidal anti-inflammatory agents; severe, even fatal, anaphylactic-like reactions have been reported THERAPEUTIC USE: Antidiabetic, Diagnostic Agent, Pituitary Function, Gastrointestinal Agent DOSE: Adult – the average daily insulin requirement in diabetes therapy ranges between 0.5 and 1.0 IU/Kg, dependent on the individual patient. CONTRA-INDICATIONS: Hypoglycaemia, hypersensitivity to human insulin or any of the excipients. ADVERSE EFFECTS: Hypoglycaemia, edema and refraction anomalies may occur, hypersensitivity reactions. THERAPEUTIC USE: Antidiabetic, Diagnostic Agent, Pituitary Function, Gastrointestinal Agent DOSE: Adult & Pedia – Diabetic ketoacidosis, initial, 0.15 unit/kg IV, followed by continuous infusion of 0.1 unit/kg/hour; if no biochemical response in 2 to 4 hours, double infusion rate; when glucose level falls to 250 mg/dL or less, halve infusion rate or add dextrose 5% infusion to maintain blood glucose between 200 and 250 mg/dL; continue until acidosis is corrected. CONTRA-INDICATIONS: hypoglycemia, systemic allergic reactions ADVERSE EFFECTS: Hypoglycemia (Severe) THERAPEUTIC USE: Antineoplastic Agent, Antiproliferative Agent, Antiviral, Immunological Agent DOSE: Adult – Type B viral hepatitis, chronic, With compensated liver disease, 5 million international units IM/SUBQ daily OR 10 million 37 PRODUCT DESCRIPTION TRADE NAME DOSAGE FORM/STRENGTH Iodine Crystals Tincture 2% Ipecacuanha (Ipecac) Solution Inhaler 20mcg Nebs. Soln.500mcg Ipratropium Bromide Atrovent Irbesartan Aprovel Tablet 150mg Tablet 300mg Iron Hydroxide Ferosac Injection 100mg/5mL Isoflurane Floran Forane Inhalation PRODUCT INFORMATION international units IM/SUBQ 3 times a week for 16 weeks. Pedia – safety and efficacy in pediatric patients less than 18 years of age have not been established other than for chronic hepatitis B. Type B viral hepatitis, chronic, With compensated liver disease, (age 1 to 17 yr) 3 million international units /m(2) SUBQ 3 times a week for the first week, then 6 million international units/m(2) SUBQ 3 times a week; total duration of therapy 16 to 24 wk; MAX 10 million international units 3 times a week CONTRA-INDICATIONS: autoimmune hepatitis, liver disease, decompensated, prior hypersensitivity to any interferon alfa preparations or components ADVERSE EFFECTS: Allergic reaction, acute, Serious, Aplastic anemia, Autoimmune disease, Cotton wool spots, Hepatotoxicity, Increased liver enzymes, Nephrotic syndrome, Neutropenia, Pancreatitis, Pneumonia, Pneumonitis, Psychotic disorder,Pulmonary infiltrate, Renal failure, Retinal hemorrhage, Retinal vascular occlusion, Thrombocytopenia THERAPEUTIC USE: Antibacterial Cleansing Agent, Antithyroid Agent, Expectorant, Iodide Supplement, Parenteral Mineral-Trace Mineral, Radiation Emergency, Thyroid Blocking Agent DOSE: Adult & Pedia – Disinfection a)2% aqueous solution TOPICALLY for minor wounds b)2% tincture TOPICALLY on intact skin c)2% in glycerin TOPICALLY on mucous membranes; Thyroid storm – 5 to 10 drops of Lugol's solution (8 mg iodide/drop) or 1 to 2 drops of saturated solution of potassium iodide (50 mg of iodide/drop) 3 times a day mixed in water or juice. CONTRA-INDICATIONS: hypersensitivity to iodine, burn patients THERAPEUTIC USE: Emetic, Nutriceutical DOSE: Adult – Poisoning, acute a)In patients 12 years of age or older, the usual dose of ipecac syrup in accidental POISONING is 30 mL orally followed by 8 or more ounces of water b)Ipecac has been used as an expectorant in doses containing approximately 0.4 to 1.2 milligrams of total alkaloids. This is equivalent to 0.3 to 1mL of ipecac syrup. Pedia – Oral – 1 to 12 years of age, In children over 1 year of age, the recommended dose of ipecac syrup is 3 teaspoonsful (15 milliliters). If vomiting does not occur within 20 minutes, a similar dose is repeated once. If the patient does not vomit within 30 minutes, the dosage should be recovered by gastric lavage CONTRA-INDICATIONS: ingestion of petroleum distillate, ingestion of strong acids or bases, ingestion of strychnine, unconsciousness or absence of gag reflex ADVERSE EFFECTS: Cardiomyopathy, Chronic use THERAPEUTIC USE: Anticholinergic, Bronchodilator, Nasal Agent DOSE: Adult – Chronic obstructive pulmonary disease a)(inhalation aerosol) 2 INHALATIONS 4 times per day; MAX 12 inhalations/day b)(inhalation solution 0.02%) 500 mcg NEBULIZED 3 to 4 times per day; separate doses by 6 to 8 hours. Pedia – Inhalation aerosol not FDAapproved for children under 12 yr of age; Chronic obstructive pulmonary disease – (inhalation solution 0.02%) 12 y of age and older, 500 mcg NEBULIZED 3 to 4 times/day per day; separate doses by 6 to 8 hours CONTRA-INDICATIONS: hypersensitivity to ipratropium products, hypersensitivity to soya lecithin or related food products ADVERSE EFFECTS: Immune hypersensitivity reaction, Angioedema, bronchospasm, urticaria, anaphylaxis, oropharyngeal edema, Paralytic ileus THERAPEUTIC USE: Angiotensin II Receptor Antagonist, Cardiovascular Agent, Renal Protective Agent DOSE: Adult – Diabetic nephropathy target maintenance dose, 300 mg ORALLY once daily Hypertension – 150 mg ORALLY once daily; may titrate to MAX of 300 mg once daily CONTRA-INDICATIONS: hypersensitivity to irbesartan products, pregnancy ADVERSE EFFECTS: Angioedema, face, lips, throat, Rhabdomyolysis THERAPEUTIC USE: Dermatological Agent, Iron Supplement, Nutriceutical, Parenteral Mineral-Trace Mineral DOSE: Adult – Iron deficiency anemia – IV or IM, dose in mL (50 mg elemental iron/mL)= 0.0442 [desired hemoglobin-observed hemoglobin] x lean body weight in kg + (0.26 x lean body wt); give 2 mL or less once daily until total dose has been reached. Pedia – Iron deficiency anemia a)children weighing over 15 kg, IV or IM, same formula as for adults b)children weighing 5-15 kg, IV or IM, dose in mL (50 mg elemental iron/mL)= 0.0442 (desired hemoglobin – observed hemoglobin) x weight (kg) + (0.26 x weight); give 2 mL or less once daily until total dose has been reached CONTRA-INDICATIONS: anemias not associated with iron deficiency, hypersensitivity to the product ADVERSE EFFECTS: Anaphylaxis, Chest pain, Dyspnea, Headache, Hematuria, Hypotension, Leukocytosis, Respiratory arrest, Seizure THERAPEUTIC USE: Haloalkane, Volatile Liquid DOSE: Adult – General anesthesia a)induction, 1.5 to 3% isoflurane with oxygen or oxygen-nitrous oxide mixture b)maintenance, 1 to 2.5% with nitrous oxide, additional 0.5 to 1% with oxygen alone. Pedia – safety in children under 2 yr of age has not been established CONTRA-INDICATIONS: hypersensitivity to isoflurane or other halogenated agents, malignant hyperthermia, known or suspected genetic susceptibility ADVERSE EFFECTS: Decreased liver function, Malignant hyperthermia, Respiratory depression 38 PRODUCT DESCRIPTION TRADE NAME DOSAGE FORM/STRENGTH Isoniazid Syrup 10mg/mL Tablet 100mg Tablet 300mg Isopropyl Alcohol Solution 70% Isoproterenol HCl (Isoprenaline) Isoproterenol Injection 1:5000 Isosorbide Dinitrate Isoket Isoket Retard Isomack Isordil SL Tablet 5mg SR Tablet 40mg Tablet 10mg Tablet 20mg Itraconazole Sporanox Capsule 100mg Ketamine HCl Ketalar Tekam Injection 50mg/mL Ketoconazole Nizoral Cream 2% Tablet 200mg L-Thyroxine Sodium Eltroxin Syrup PRODUCT INFORMATION THERAPEUTIC USE: Antitubercular, Isonicotinic Acid DOSE: Adult – Tuberculosis, 5 mg/kg ORALLY/IV/IM once daily (MAX 300 mg/dose) OR 15 mg/kg ORALLY/IV/IM 1 to 3 times per week (MAX 900 mg/dose) in combination with other antitubercular drugs. Pedia – Tuberculosis, 10 to 15 mg/kg ORALLY daily (MAX 300 mg/dose) OR 20 to 30 mg/kg ORALLY twice per week (MAX dose 900 mg) in combination with other antitubercular drugs CONTRA-INDICATIONS: drug fever, chills, arthritis, or other adverse reaction to isoniazid, hepatic injury due to isoniazid, hypersensitivity to isoniazid, including drug-induced hepatitis, liver disease, acute ADVERSE EFFECTS: Agranulocytosis, Anemia, Megaloblastic anemia, Seizure, Systemic lupus erythematosus, Thrombocytopenia THERAPEUTIC USE: Antibacterial Cleansing Agent, Toxicology-Antidote Agent CONTRA-INDICATIONS: Patients with epilepsy, urinary tract infections, and those with prior addiction, ETHANOL in DEXTROSE solutions should not be used in diabetic coma THERAPEUTIC USE: Adrenergic, Bronchodilator, Sympathomimetic, Vasopressor DOSE: Adult – Cardiac arrest a)IM, 0.2 mg, followed by 0.02 to 1 mg, depending on response b)IV, 2 to 10 mcg/min, titrate to response c)SC, 0.2 mg, followed by 0.15 to 0.2 mg, depending on response CONTRA-INDICATIONS: angina pectoris, digitalis-induced tachycardia or heart block, hypersensitivity to isoproterenol products, tachyarrhythmias ADVERSE EFFECTS: Coronary arteriosclerosis THERAPEUTIC USE: Antianginal, Coronary Vasodilator, Nitrate DOSE: Adult – Angina; Prophylaxis a)ORAL (immediate release forms), initial 5 to 20 mg ORALLY 2 to 3 times daily; maintenance 10 to 40 mg ORALLY 2 to 3 times daily; a daily dose-free interval of at least 14 h is recommended to minimize tolerance b)SUBLINGUAL, 2.5 to 5 mg SL 15 min before expected activity. Pedia – safety and efficacy not established in pediatric patients CONTRA-INDICATIONS: hypersensitivity to organic nitrates ADVERSE EFFECTS: Methemoglobinemia, Rebound hypertension, Syncope, Unstable angina THERAPEUTIC USE: Antifungal, Triazole DOSE: Adult – Aspergillosis a)oral, 200mg to 400mg daily b)IV, 200mg infused over one hour twice daily for 4 doses, then 200 mg once daily c)life-threatening situations, oral, 200mg 3 times daily for 3 days, then oral 200 mg daily; treatment should continue for at least 3 months d)life-threatening situations, IV, 200 mg 2daily for 4 doses, then IV 200 mg daily; continue for at least 3 months. Pedia – Limited data: oral solution, 6 months-12 yr, 5mg/kg/day for 2 wk; oral capsule, 3-16 yr, 100 mg/day CONTRA-INDICATIONS: Coadministration with cisapride, dofetilide, oral midazolam, pimozide, levacetylmethadol (levomethadyl), quinidine, lovastatin, simvastatin, or triazolam, Coadministration with ergot alkaloids metabolized by CYP3A4, such as dihydroergotamine, ergometrine (ergonovine), ergotamine and methylergometrine (methylergonovine), Congestive heart failure or history of congestive heart failure (itraconazole capsules for treatment of onychomycosis),Pregnant women or women contemplating pregnancy (itraconazole capsules for treatment of onychomycosis),Previous hypersensitivity to itraconazole ADVERSE EFFECTS: Anaphylaxis, Congestive heart failure, IV, Hepatotoxicity, Neutropenic disorder, Stevens-Johnson syndrome THERAPEUTIC USE: Anesthetic Adjunct DOSE: Adult – General anesthesia; Adjunct a)induction, 1 to 4.5 mg (base)/kg IV single dose b)induction, 1 to 2 mg/kg IV infusion at 0.5 mg/kg/min; in addition to diazepam 2-5 mg IV over 1 min c)induction, 6.5 to 13 mg (base)/kg IM d)maintenance, 0.1 to 0.5 mg (base)/min IV infusion, repeat as needed; augmented with diazepam 2-5 mg IV e)maintenance, 0.01 to 0.03 mg/kg/min continuous IV infusion f)maintenance, increments of one-half to the full induction dose may be repeated as needed. Pedia – not FDA approved in children under age 16 yr, General anesthesia; Adjunct a)induction, 5 to 10 mg (base)/kg IM; range, 4 to 13 mg/kg b)induction, 1 to 2 mg/kg IV; range, 0.5-4.5 mg/kg c)maintenance, 0.01 to 0.03 mg/kg/min continuous IV infusion d)maintenance, increments of one-half to the full induction dose may be repeated as needed CONTRA-INDICATIONS: hypersensitivity to ketamine products, conditions where hypertension is hazardous ADVERSE EFFECTS: Increased blood pressure, Increased muscle function, Respiratory depression, Tachyarrhythmia THERAPEUTIC USE: Antifungal, Antiseborrheic, Imidazole, Steroid Synthesis Inhibitor DOSE: Adult – Candidal vulvovaginitis, 200 to 400 mg ORALLY once daily; minimum treatment for candidiasis is 1-2 weeks; Candidiasis of skin – apply 2% cream TOPICALLY once daily for 2 weeks. Pedia – Candidal vulvovaginitis – age 2 yr and older, 3.3 to 6.6 mg/kg ORALLY once daily; minimum treatment for candidiasis is 1-2 weeks CONTRA-INDICATIONS: hypersensitivity to ketoconazole products, concurrent use with astemizole or terfenadine, concurrent use with cisapride or oral triazolam ADVERSE EFFECTS: Anaphylaxis, Hepatotoxicity THERAPEUTIC USE: Diagnostic Agent, Thyroid Function, Thyroid Supplement DOSE: Adult – Hypothyroidism a)1.7 mcg/kg/day ORALLY in a single daily dose (usual maintenance dose is 100-125 mcg/day 70 kg adult) 39 PRODUCT DESCRIPTION TRADE NAME DOSAGE FORM/STRENGTH 25mcg/mL Tablet 25 mcg Tablet 50mcg Tablet 100mcg Labetalol Trandate Lactose Lactulose Inj. 5mg/mL Syrup 10mg/mL Tablet 100mg Tablet 200mg Powder 50grams Duphalac Rialac Syrup Latanoprost Xalatan Eye Drops 50mcg/mL Letrozole Femara Tablet 2.5mg Levetiracetam Keppra Tablet 500mg Levobunolol HCl Betagan Eye Drops 0.5% PRODUCT INFORMATION b)severe hypothyroidism, initial: 12.5 to 25 mcg/day with increases of 25 mcg/day every 2 to 4 weeks. Pedia - Hypothyroidism a)(0 to 3 months) 10 to 15mcg/kg/day b)(3 to 6 months) 8 to 10mcg/kg/day c)(6 to 12 months) 6 to 8mcg/kg/day d)(1 to 5 yrs) 5 to 6mcg/kg/day e)(6 to 12 yrs) 4 to 5mcg/kg/day f)(over 12 yrs, growth and puberty incomplete) 2 to 3 mcg/kg/day g)(growth and puberty complete) 1.7 mcg/kg/day CONTRA-INDICATIONS: acute MI, hypersensitivity to thyroid hormone, treatment of obesity, uncorrected adrenal cortical insufficiency, untreated angina, untreated hypertension, untreated thyrotoxicosis ADVERSE EFFECTS: Myocardial infarction, Osteopenia, Pseudotumor cerebri, Seizure THERAPEUTIC USE: Alpha/Beta-Adrenergic Blocker, Antianginal, Antihypertensive DOSE: Adult – Hypertension a)initial, 100 mg ORALLY twice daily b)titration, may increase dose in increments of 100 mg ORALLY twice daily every 2-3 days c)maintenance, 200-400 mg ORALLY twice daily d)IV to ORAL conversion, initial, 200 mg ORALLY, followed in 6-12hr by 200-400 mg ORALLY depending on BP response. Pedia – Not FDA approved in children. Hypertension a)initial, 1-3 mg/kg/day ORALLY in 2 divided doses; MAX 10-12 mg/kg/day ORALLY up to 1200 mg/day in 2 divided doses b)ages 3-15yr, initial bolus, 0.2-1 mg/kg IV c)ages 315yr, continuous infusion, 0.25-1.5 mg/kg/hr IV CONTRA-INDICATIONS: bronchial asthma or chronic obstructive pulmonary disease, cardiogenic shock, conditions associated with severe and prolonged hypotension, hypersensitivity to labetalol, overt cardiac failure, second and third degree AV block, severe sinus bradycardia ADVERSE EFFECTS: Bronchospasm, Hepatotoxicity (Severe), Hyperkalemia, Ventricular arrhythmia THERAPEUTIC USE: Widely used in pharmaceutical manufacturing. In the production of capsules or tablets it may be employed as a diluent, bulking agent, filler or excipient and in powders as a bulking agent. ADVERSE EFFECTS: Lactose intolerance occurs due to a deficiency of the intestinal enzyme lactase. Ingestion of lactose by patients with lactase deficiency leads to a clinical syndrome of abdominal pain, diarrhea, distension and flatulence. THERAPEUTIC USE: Gastrointestinal Agent, Laxative, Hyperosmotic DOSE: Adult – Constipation, 15 to 45 mL (20 grams/30 mL) ORALLY 3 to 4 times daily for 24 to 48 hours; MAX 40 grams/day; Hepatic encephalopathy; Treatment and Prophylaxis, initially, 30 to 45 mL (20 grams/30 mL) ORALLY 3 to 4 times daily, then adjusted every 1 to 2 days to achieve 2 to 3 soft formed stools/day or 300 mL (200 grams) mixed with 700 mL of water or saline RECTALLY as a retention enema (retain for 30 to 60 min) every 4 to 6 hours as needed. Pedia – Constipation a)(infants) 2.5 to 10 mL/day (20 grams/30 mL) ORALLY in 3 to 4 divided doses b)(children) 40 to 90 mL/day (20 grams/30 mL) ORALLY in 3 to 4 divided doses CONTRA-INDICATIONS: hypersensitivity to lactulose products, galactosemia, patients requiring a galactose free diet ADVERSE EFFECTS: Hypernatremia, Hypokalemia THERAPEUTIC USE: Antiglaucoma, Prostaglandin DOSE: Adult – Ocular hypertension – Raised intraocular pressure, 1 drop in affected eye(s) once daily, in the evening; Open-angle glaucoma – Raised intraocular pressure, 1 drop in affected eye(s) once daily, in the evening CONTRA-INDICATIONS: hypersensitivity to latanoprost or benzalkonium chloride ADVERSE EFFECTS: Macular retinal edema THERAPEUTIC USE: Antineoplastic Agent, Aromatase Inhibitor, Endocrine-Metabolic Agent DOSE: Adult – Breast cancer, Neoadjuvant, postmenopausal, hormone receptor-positive, 2.5 mg ORALLY once daily for 3 to 8 months prior to surgery. Pedia – safety and effectiveness not established in children CONTRA-INDICATIONS: hypersensitivity to letrozole or any component of the product, premenopausal endocrine status; potential for maternal and fetal toxicity and fetal malformations ADVERSE EFFECTS: Fracture of bone, Heart failure, Myocardial infarction, Pancytopenia, Pleural effusion, Pulmonary embolism, Thromboembolic disorder THERAPEUTIC USE: Anticonvulsant DOSE: Adult – Myoclonic seizure; Adjunct, initial, 500 mg ORALLY twice daily; increase daily doses by 1000 mg every 2 weeks to reach a target dose of 3000 mg/day. Pedia – (tablets, oral solution) safety and efficacy not established in children less than 4 years of age. Partial seizure; Adjunct, (4 -15 years of age) 10 mg/kg ORALLY twice daily; may increase dosage by 20 mg/kg/day in 2 divided doses every 2 weeks to a MAX of 60 mg/kg/day (in 2 divided doses) ADVERSE EFFECTS: Suicidal intent, Suicide THERAPEUTIC USE: Antiglaucoma, Beta-Adrenergic Blocker, Nonselective DOSE: Adult – Raised intraocular pressure, In patients with open-angle glaucoma or ocular hypertension a)(0.25% solution) 1-2 drops in affected eye(s) twice daily b)(0.5% solution) 1-2 drops in affected eye(s) once daily; MAX 1 drop twice a day. Pedia – Safety and efficacy not established in children CONTRA-INDICATIONS: bronchial asthma or chronic obstructive pulmonary disease, cardiogenic shock, hypersensitivity to levobunolol, overt cardiac failure, second and third degree AV block, severe sinus bradycardia 40 PRODUCT DESCRIPTION TRADE NAME DOSAGE FORM/STRENGTH Levodopa/Carbidopa Sinemet Tablet 110mg Tablet 275mg Levosimendan Simdax Inj. 2.5mg/mL Lidocaine HCl Lidocaine /Hydrocortisone Xylocaine Xylocard Xyloproct Liquid Parafin Ointment Injection 1% Injection 2% Lidocaine/Adrenaline Lidocaine/Prilocaine Gel 2% Injection 1% Injection 2% Injection 20% Ointment 5% Spray 10% Syringe 2% Viscous 2% Emla Lacri – Lube Mineral Oil Cream 5% Eye Ointment Oil 60mL PRODUCT INFORMATION THERAPEUTIC USE: Antiparkinsonian DOSE: Adult – Parkinson's disease, Idiopathic a)25/100 TAB; initial, 1 TAB ORALLY 3 times a day; increase by 1 TAB daily or every other day to 8 TABS daily b)10/100 TAB; initial, 1 TAB ORALLY 3 or 4 times daily; increase by 1 TAB daily or every other day to 8 TABS daily c)50/200 (sustained release) TAB, 1 TAB ORALLY twice daily at an interval of at least 6 hr d)(sustained release) titration, doses and dosing intervals may be increased or decreased depending upon therapeutic response; most patients adequately treated with doses that provide 400-1600 mg of levodopa per day, administered as divided doses at intervals of 4-8 hr during the waking day; dosage adjustment interval at least 3 days. Pedia – safety and efficacy not established in pediatric patients CONTRA-INDICATIONS: hypersensitivity to levodopa/carbidopa products, history of melanoma, undiagnosed skin lesions, narrow-angle glaucoma, nonselective MAO inhibitors concurrently or less than 2 wks prior ADVERSE EFFECTS: Dyskinesia, Heart disease, Orthostatic hypotension, Psychotic disorder DOSE: Intravenous route a)In a dose-ranging, comparative pharmacodynamic study involving patients with CONGESTIVE HEART FAILURE, the optimal dosing regimen was determined to be an initial bolus of levosimendan 6 to 24 micrograms/kilogram (mcg/kg) delivered over 10 minutes, followed by a continuous infusion of levosimendan 0.05 to 0.2 mcg/kg/minute b)A continuous infusion of levosimendan 0.1 microgram/kilogram/minute improved hemodynamics in a small series (n=10) of critically ill patients with CARDIOGENIC SHOCK requiring catecholamines. No bolus dose was given CONTRA-INDICATIONS: Prior hypersensitivity to levosimendan or racemic simendan THERAPEUTIC USE: Amino Amide, Anesthetic, Local, Antiarrhythmic, Group IB DOSE: Adult – Local anesthetic intravenous regional block a)10 to 60 mL of a 0.5% solution IV for a total dose of 50 to 300 mg b)the maximum individual dose should not exceed 4 mg/kg and the maximum total dose of 300 mg should not be exceeded Local anesthetic intravenous regional block CONTRA-INDICATIONS: sensitivity to local anesthetics of the amide type or to any other component of the product ADVERSE EFFECTS: Cardiac arrest, Cardiac dysrhythmia, Heart block, Loss of consciousness, Methemoglobinemia, Seizure, Tremor THERAPEUTIC USE: For the treatment of pain, itching and discomfort arising from irritated anorectal tissues e.g. haemorrhoids, pruritus ani, proctitis, milder forms of anal fissures, postoperative pain relief. DOSE: Apply the ointment several times a day in a thin layer to the affected area. Intrarectal use, apply the ointment with the special applicator. Cleanse the applicator thoroughly after use. A daily dose of 6grams of ointment is well within safety limits. The duration of treatment may vary between ten day and three weeks. CONTRA-INDICATIONS: Known hypersensitivity to local anesthetics of the amide type or to other components of the product. For infections caused by viruses, bacteria, pathogenic fungi or parasites, topical glucocorticoids should not be used without concomitant causal therapy. ADVERSE EFFECTS: Allergic reactions THERAPEUTIC USE: Local infiltration anaesthesia, Regional anaesthesia, Intra or Peri-articular infiltration, Sympathetic infiltration DOSE: Adult – the maximum dose should not exceed 500mg, when using epidural anesthesia in obstetric it is advisable to reduce the dose by one-half. Pedia – An adrenaline concentration of 1/400,000 should not be exceeded. It is therefore necessary to dilute formulations containing Adrenaline. THERAPEUTIC USE: Anesthetic, Amino Amide Combination, Anesthetic, Local DOSE: Adult – Topical local anesthetic, Genital mucous membranes, females, apply a thick layer (5-10 g) of CREAM for 5-10 min, perform local anesthetic infiltration immediately after removal of cream. Pedia – Topical local anesthetic to skin a)(age 0-3 months or weight <5 kg) MAX dose, 1 g; MAX application area, 10 cm(2); MAX application time, 1 hr b)(age 3-12 months, weight >5 kg) MAX dose, 2 g MAX application area, 20 cm(2); MAX application time, 4 hr c)(age 1-6 yr, weight >10 kg) MAX dose, 10 g MAX application area, 100 cm(2); MAX application time, 4 hr d)(age 7-12 yr, weight >20 kg) MAX dose, 20 g; MAX application area, 200 cm(2); MAX application time, 4 hr CONTRA-INDICATIONS: hypersensitivity to local amide-type anesthetics or to any component of the product ADVERSE EFFECTS: Central nervous system finding, Depression/excitation, Methemoglobinemia, Myocardial dysfunction, Seizure THERAPEUTIC USE: Emollient, Laxative DOSE: Adult – Constipation a)15 to 45 mL ORALLY once daily at bedtime, MAX 45 mL b)enema: empty contents of 1 bottle (133 mL) into RECTUM once; Dry skin, apply to damp skin as needed. Pedia – Constipation a)(5 to 11 yr) 5 to 15 mL ORALLY once daily at bedtime b)(12 yr and older) enema: empty contents of 1 bottle (133 mL) into RECTUM once c)(2 to 11 yr) enema: empty contents of 1-half bottle (66.5 mL) into RECTUM once; Dry skin, apply to skin directly after bath 41 PRODUCT DESCRIPTION TRADE NAME Loperamide HCl Imodium Loratadine Clara Claritin Loratan Lorine DOSAGE FORM/STRENGTH Capsule 2mg Syrup 5mg/5mL Tablet 10mg Lorazepam Ativan Inj. 4mg/2mL Tablet 1mg Tablet 2mg Macrogols /Polyethylene Glycol Cololyt Granules Magnesium Citrate Epimag Powder 5grams Magnesium Sulphate Injection 10% Injection 50% Powder Syrup 100mg/mL PRODUCT INFORMATION CONTRA-INDICATIONS: appendicitis, children less than 2 yr of age, children less than 6 yr of age, colostomy/ileostomy, diverticulitis, hypersensitivity to mineral oil products, ulcerative colitis, rectal bleeding ADVERSE EFFECTS: Pneumonitis due to inhalation of oil or essence, Rectal hemorrhage THERAPEUTIC USE: Antidiarrheal DOSE: Adult – Diarrhea, acute, 4 mg ORALLY followed by 2 mg after each loose stool up to a maximum of 16 mg/day. Pedia – Diarrhea, acute a)first day dosage; (2 to 5 y, 13 to 20 kg) 1 mg ORALLY 3 times daily b)first day dosage; (6 to 8 y, 20 to 30 kg) 2 mg ORALLY twice daily c)first day dosage; (8 to 12 y, greater than 30 kg) 2 mg ORALLY 3 times daily d)subsequent daily dosage; (2 to 12 y) 1mg/10kg of body weight ORALLY only after a loose stool, total daily dose should not exceed dosages for the first day CONTRA-INDICATIONS: abdominal pain in the absence of diarrhea, bacterial enterocolitis, caused by invasive organisms including Salmonella, Shigella, and Campylobacter; do not use as primary therapy, dysentery, acute; do not use as primary therapy, hypersensitivity to loperamide or to any of the excipients, infants below 24 months of age, pseudomembranous colitis, associated with the use of broad spectrum antibiotics; do not use as primary therapy, ulcerative colitis, acute; do not use as primary therapy ADVERSE EFFECTS: Necrotizing enterocolitis in fetus OR newborn THERAPEUTIC USE: Antihistamine, Less-Sedating, Piperidine, Respiratory Agent DOSE: Adult – Asthma (allergic asthma) 10 to 20 mg ORALLY once daily has been used for up to 8 weeks. Pedia – Seasonal allergic rhinitis a)(2 to 5 y) 5 mg ORALLY once daily b)(6 y and older) 10 mg orally once daily CONTRA-INDICATIONS: hypersensitivity to loratadine or any of its ingredients ADVERSE EFFECTS: Decreased liver function THERAPEUTIC USE: Antianxiety, Anticonvulsant, Benzodiazepine, Short or Intermediate Acting, Skeletal Muscle Relaxant DOSE: Adult – Anxiety a)initial, 2 to 3 mg/day ORALLY divided into 2 to 3 daily doses b)maintenance, 2 to 6 mg/day ORALLY divided into 2 to 3 daily doses; MAX dose 10 mg/day. Pedia – Safety and effectiveness of lorazepam tablets in children less than 12 years old have not been established. Safety and effectiveness of lorazepam injection in children less than 18 years old have not been established. Status epilepticus 0.05 to 0.1 mg/kg IV (MAX 4 mg/dose) CONTRA-INDICATIONS: hypersensitivity to benzodiazepines or any component of the product (oral and injection), polyethylene glycol, propylene glycol, or benzyl alcohol, intra-arterial administration; may produce arteriospasm resulting in gangrene, narrow-angle glaucoma, acute, respiratory insufficiency, severe; in the absence of resuscitative equipment, sleep apnea syndrome. ADVERSE EFFECTS: Acidosis THERAPEUTIC USE: Indicated for cleansing the bowel by means of total gut perfusion and whole bowel irrigation in preparation for gastrointestinal examination and surgery. DOSE: The typical dosage per patient is 3 to 4 liters. The total volume can be drunk on the eve of the examination, especially before double contrast enemas. Alternatively the dose can be divided into 1 liter on the eve and 2liters in the morning of the day of examination. Recommended ingestion rate ranges from 1.2 liters to 1.8 liters per hour (200 to 300mL every 10minutes). It should preferably be drunk cool. The addition of sugarless flavorings or lemon juice is possible. Patients should fast for three or four hours before ingestion begins. Patients should never be given solid food less than two hours prior to administration. ADVERSE EFFECTS: Nausea and flatulence, vomiting, rectal irritation. THERAPEUTIC USE: Antacid, Magnesium Containing, Anticonvulsant, Anti-Inflammatory, Laxative, Hyperosmotic & Stimulant, Magnesium Supplement, Nutriceutical, Parenteral Mineral-Trace Mineral, Renal-Urologic Agent DOSE: Adult – Hypomagnesemia; Prophylaxis, 400 mg ORALLY daily with meals; Preparation of bowel for procedure – 150 to 300 mL (1.745 g/30 mL solution) ORALLY once, may repeat as needed. Pedia – Preparation of bowel for procedure, (6-12 yr): 0.5 mL/kg (1.745 g/30 mL solution) up to a maximum of 200 mL every 4-6 hours until stools are clear CONTRA-INDICATIONS: abdominal pain, nausea/vomiting, rectal bleeding, heart block, low-salt diet, severe renal disease ADVERSE EFFECTS: Hypermagnesemia, Hypoventilation DOSE: Adult – Hypomagnesemia; Treatment and Prophylaxis a)mild hypomagnesemia; 1 g (magnesium sulfate 50% solution) IM every 6 hours for 4 doses b)severe hypomagnesemia; up to 250 mg/kg (magnesium sulfate 50% solution) IM in 4 hours OR 5 g in 1 L of D5W or NS by slow IV infusion over 3 hours c)TPN, maintenance; 1 to 3 g (8 to 24 mEq) magnesium sulfate daily given parenterally. Pedia – Hypomagnesemia; Treatment and Prophylaxis a)neonates, magnesium sulfate 25 to 50 mg/kg IV every 8 to 12 hours for 2 to 3 doses b)children, magnesium sulfate 25 to 50 mg/kg IV every 4 to 6 hours for 3 to 4 doses; MAX single dose is 2000 mg c)TPN, maintenance; infants; 0.25 to 1.25 g (2 to 10 mEq) magnesium sulfate daily given parenterally CONTRA-INDICATIONS: heart block, parenteral administration, myocardial damage, parenteral administration ADVERSE EFFECTS: Abnormal ECG, Blood coagulation disorder with prolonged bleeding time, Central nervous system depression, Heart block, Hyporeflexia, Hypotension, Respiratory tract paralysis, Vasodilatation 42 PRODUCT DESCRIPTION Maprotiline HCl TRADE NAME Ludiomil Measles/Mumps /Rubella Vaccine DOSAGE FORM/STRENGTH Tablet 10mg Tablet 25mg Injection Medazole Mebendazole Vermox Susp. 20mg/mL Tablet 100mg Wormazol Medroxyprogesterone Acetate Provera Tablet 5mg Fenam Mefenamic Acid Fendol Mafepain Capsule 250mg Syrup 50mg/5mL Ponstan Megestrol Acetate Megace Tablet 40mg PRODUCT INFORMATION THERAPEUTIC USE: Antidepressant Tetracyclic DOSE: Adult – Bipolar disorder, depressed phase a)outpatients: 75 mg/day ORALLY (2-3 divided doses) for 2 weeks; may increase in 25 mg increments up to a MAX of 225 mg/day in single or divided doses b)inpatients: 100-150 mg/day ORALLY (2-3 divided doses) for 2 weeks; may increase in 25 mg increments up to a MAX of 225 mg/day in single or divided doses c)maintenance: 75-150 mg/day ORALLY in single or divided doses. Pedia – safety and efficacy have not been established in patients below 18 years of age CONTRA-INDICATIONS: coadministration with a MAOI or use within 14 days of discontinuing a MAOI, hypersensitivity to maprotiline hydrochloride, myocardial infarction (MI), during the acute recovery period, seizure disorder ADVERSE EFFECTS: Decreased liver function, Depression, Worsening, Granulocytopenic disorder, Jaundice, Mania, Neutropenia, Seizure, Suicidal thoughts, Suicide THERAPEUTIC USE: Vaccine DOSE: Adult – Measles-mumps-rubella vaccination, 0.5 mL SC. Pedia – Safety and effectiveness of measles vaccine in infants below the age of 6 months have not been established. Safety and effectiveness of mumps and rubella vaccine in infants less than 12 months of age have not been established. Measles-mumps-rubella vaccination 0.5 mL SC at 12 to 15 months and at 4 to 6 years of age CONTRA-INDICATIONS: alkylating agents, antimetabolites, cerebrospinal fluid leaks, clinical judgment with respect to immunosuppressive treatment is indicated (eg, in general, short-term steroid therapy or alternate-day therapy are not significantly immunosuppressive, and there is no contraindication in these settings), corticosteroid use in large amounts immunodeficiency, congenital, leukemia, lymphoma, or generalized malignancy, patients with active, untreated tuberculosis (potential exacerbation), patients with moderate-to-severe immunodeficiency conditions, including those receiving immunosuppressive therapy (potential for viral replication and/or reduced vaccine efficacy); HIV-infected patients can be immunized if they are not severely immunosuppressed; leukemia patients in remission can be vaccinated if they have not received chemotherapy in the previous three months, pregnancy should be avoided for 4 weeks; pregnancy should be avoided for 3 months postvaccination based on manufacturer recommendations, prior hypersensitivity to any component of combined measles, mumps, and rubella vaccines, including gelatin: Neomycin is component of MMR-II®; neomycin sensitivity is a contraindication only in patients with a history of anaphylaxis following topical or systemic use; a history of contact dermatitis is not a contraindication, radiation therapy, severe febrile illness ADVERSE EFFECTS: Anaphylaxis, Encephalitis, Guillain-Barre syndrome, Association with vaccine unlikely, Optic neuritis, Polyneuritis, Polyneuropathy, Stevens-Johnson syndrome, Thrombocytopenia THERAPEUTIC USE: Anthelmintic, Benzimidazole DOSE: Adult – Ancylostomiasis and necatoriasis, 100 mg ORALLY twice daily for 3 consecutive days; drug regimen may be repeated in 3 wks if necessary. Pedia – Ancylostomiasis and necatoriasis, (over 2 y) 100 mg ORALLY twice daily for 3 consecutive days; drug regimen may be repeated in 3 wks if necessary CONTRA-INDICATIONS: hypersensitivity to mebendazole products ADVERSE EFFECTS: Hepatitis, Seizure THERAPEUTIC USE: Antineoplastic Agent, Contraceptive, Progestin, Endocrine-Metabolic Agent DOSE: Adult – Abnormal uterine bleeding unrelated to menstrual cycle, Hormonal imbalance-induced, 5-10 mg ORALLY daily for 5-10 days beginning on day 16 or 21 of the menstrual cycle; to produce optimum secretory transformation of the primed endometrium, 10 mg ORALLY daily for 10 days beginning on day 16 of the cycle. Pedia – Safety and efficacy not established in children CONTRA-INDICATIONS: as a diagnostic test for pregnancy, malignancy of breast or genital organs, pregnancy, sensitivity to medroxyprogesterone acetate or any of its ingredients, liver dysfunction or disease, missed abortion, thrombophlebitis, thromboembolic disorders, cerebral apoplexy, or a history of these conditions, undiagnosed vaginal bleeding ADVERSE EFFECTS: Anaphylaxis, Decreased bone mineral density, Deep venous thrombosis, Jaundice, Osteoporosis, Pulmonary embolism, Thrombophlebitis THERAPEUTIC USE: Analgesic, Antimigraine, Central Nervous System Agent, Fenamate, NSAID DOSE: Adult – Pain, initial dose 500 mg, followed by 250 mg every 6 hours as needed, usually not longer than 1 week. Pedia – safety and efficacy for pediatric patients younger than 14 years have not been established CONTRA-INDICATIONS: treatment of peri-operative pain in setting of coronary artery bypass graft, hypersensitivity to mefenamic acid, patients who have experienced asthma, urticaria, or allergic-type reactions after taking aspirin or other nonsteroidal anti-inflammatory agents; severe, even fatal, anaphylactic-like reactions have been reported ADVERSE EFFECTS: Acute renal failure, Bronchospasm, Cerebrovascular accident, Gastrointestinal perforation, Gastrointestinal ulcer, Hepatitis, Hypertension, Inflammatory disorder of digestive tract, Jaundice, Liver failure, Myocardial infarction, Scaling eczema, StevensJohnson syndrome, Thrombotic tendency observations, Toxic epidermal necrolysis THERAPEUTIC USE: Endocrine-Metabolic Agent, Progestin DOSE: Adult – Breast cancer, palliative treatment of advanced disease (recurrent, inoperable, or metastatic), 40 mg ORALLY FOUR times 43 PRODUCT DESCRIPTION TRADE NAME DOSAGE FORM/STRENGTH Tablet 160mg Meloxicam Coxicam Mobic Moven Supp. 15mg Tablet 7.5mg Tablet 15mg Melphalan Alkeran Tablet 2mg Meningococcal Vaccine ACWY Injection Mercaptopurine Puri – Nethol Tablet 50mg Meropenem Meronem Injection 500mg Injection 1gram Asacol Mesalazine Pentasa Salofalk SR Tablet 500mg PRODUCT INFORMATION daily. Pedia – safety and effectiveness not established in children CONTRA-INDICATIONS: history of hypersensitivity to megestrol acetate or any component of the formulation, known or suspected pregnancy ADVERSE EFFECTS: Adrenal insufficiency, Anemia, Deep venous thrombosis, Pulmonary embolism, Thrombophlebitis THERAPEUTIC USE: Analgesic, Antirheumatic, Central Nervous System Agent, Musculoskeletal Agent, NSAID, Oxicam DOSE: Adult – Rheumatoid arthritis, 7.5 mg ORALLY once a day, MAX 15 mg ORALLY once a day. Pedia – safety and efficacy in children less than 18 years old have not been established. Juvenile rheumatoid arthritis, polyarticular – Pauciarticular juvenile rheumatoid arthritis, (2 years and older) 0.125 mg/kg ORALLY once daily; MAX 7.5 mg daily; dosing should be individualized based on the weight of the child. CONTRA-INDICATIONS: treatment of peri-operative pain in setting of coronary artery bypass graft, hypersensitivity to meloxicam, patients who have experienced asthma, urticaria, or allergic-type reactions after taking aspirin or other nonsteroidal anti-inflammatory agents; severe, even fatal, anaphylactic-like reactions have been reported THERAPEUTIC USE: Alkylating Agent, Antineoplastic Agent, Nitrogen Mustard, DOSE : Adult – Multiple myeloma, Palliative treatment, 6 mg/day ORALLY for 2 to 3 wk, then 4 wk off, then restart at 2 mg/day when white blood cell and platelet counts are rising; other regimens include 0.25 mg/kg/day for 4 consecutive days (0.2 mg/kg/day for 5 days) in combination with prednisone- total dose of 1 mg/kg/course repeated every 4 to 6 weeks if granulocyte/platelet counts have returned to normal; 10 mg/day for 7 to 10 days with a maintenance dose of 2 mg/day; 0.15 mg/kg/day for 7 days with a maintenance dose of 0.05 mg/kg/day or less. Pedia – safety & efficacy have not been established in pediatric patients CONTRA-INDICATIONS: Hypersensitivity to melphalan products, Previous resistance to the drug ADVERSE EFFECTS: Anaphylaxis, Hemolytic anemia, Hepatitis, Immune hypersensitivity reaction, Jaundice, Liver function tests abnormal, Myelosuppression, Pulmonary fibrosis Pulmonary infiltrate, Secondary malignant neoplastic disease, Sterility, Vasculitis THERAPEUTIC USE: Vaccine DOSE: Adult – Meningococcal infectious disease; Prophylaxis, primary or revaccination, 0.5 mL SUBQ as a single dose. Pedia – safety and effectiveness in children below the age of 2 y have not been established; Meningococcal infectious disease; Prophylaxis (2 y and older) primary or revaccination, 0.5 mL SUBQ as a single dose CONTRA-INDICATIONS: Any acute illness, Sensitivity to thimerosal or any other component of the vaccine THERAPEUTIC USE: Antimetabolite, Antineoplastic Agent, Antirheumatic, Cytotoxic, Gastrointestinal Agent DOSE: Adult – Acute lymphoid leukemia, As induction and maintenance therapy a)induction, 2.5 mg/kg/day (100-200 mg) ORALLY; if no clinical improvement after 4 weeks, may increase dose to 5 mg/kg daily b)maintenance, 1.5-2.5 mg/kg/day ORALLY as single dose; usually used in combination with methotrexate. Pedia – Acute lymphoid leukemia, As induction and maintenance therapy a)induction, 2.5 mg/kg/day ORALLY; if no clinical improvement after 4 weeks, may increase dose to 5 mg/kg daily b)maintenance, 1.5-2.5 mg/kg/day ORALLY; usually used in combination with methotrexate CONTRA-INDICATIONS: hypersensitivity to mercaptopurine/component of formulation, prior resistance to mercaptopurine or thioguanine, should not be used unless acute leukemia diagnosis is established ADVERSE EFFECTS: Hepatotoxicity, Hyperuricemia, Myelosuppression, Ulceration of intestine THERAPEUTIC USE: Antibiotic, Beta-Lactam, Carbapenem DOSE: Adult – Infectious disease of abdomen, Complicated, 1 g IV injection or infusion every 8 h. Pedia – Bacterial meningitis, 3 mo and older, 40 mg/kg IV injection or infusion every 8 h, MAX dose 2 g every 8 h; Infection of skin &/or subcutaneous tissue, Complicated 3 mo and older, 10 mg/kg IV infusion or injection every 8 h; MAX dose 500 mg every 8 h CONTRA-INDICATIONS: hypersensitivity to meropenem or any component of its product, other drugs in the same class (carbapenems), or other beta-lactam antibiotics ADVERSE EFFECTS: Agranulocytosis, Angioedema, Erythema multiforme, Leukopenia, Neutropenia, Seizure, Most commonly in patients with CNS disorders, Stevens-Johnson syndrome, Toxic epidermal necrolysis THERAPEUTIC USE: Anti-Inflammatory, Gastrointestinal Agent, Salicylate, Non-Aspirin DOSE: Adult - Ulcerative colitis, Active a)(controlled-release capsule) 1 gram ORALLY 4 times daily for up to 8 wks b)(Asacol® delayedrelease tablet) 800 mg ORALLY 3 times daily for 6 wks. Pedia – safety & efficacy has not been established in pediatric patients CONTRA-INDICATIONS: hypersensitivity to mesalamine salicylates (including aspirin), or to any component of the tablets, capsules, rectal suspension, or rectal suppositories, including the suppository vehicle (saturated vegetable fatty acid esters ADVERSE EFFECTS: Agranulocytosis, Aplastic anemia, Hepatotoxicity, Leukopenia, Neutropenia, Pancreatitis, Pancytopenia, Pericarditis, Renal impairment, Thrombocytopenia 44 PRODUCT DESCRIPTION Mesna TRADE NAME Uromitexan DOSAGE FORM/STRENGTH Injection 100mg/mL Formit Metformin Glucophage Tablet 500mg Tablet 850mg Metfor Methotrexate Inj. 50mg/5mL Inj. 500mg/5mL Intrathecal 5mg Tablet 2.5mg Methylcellulose Powder Solution 1% Methyldopa Methylene Blue Aldomet Syrup 25mg/mL Tablet 250mg Injection 1% PRODUCT INFORMATION THERAPEUTIC USE: Hemorrhagic Cystitis Inhibitor DOSE: Adult – Hemorrhagic cystitis, Ifosfamide-induced; Prophylaxis – bolus IV regimen, IV bolus 20% of ifosfamide dose (w/w) at 0, 4, 8 h. Pedia – safety and efficacy of mesna tablets have no been established in children CONTRA-INDICATIONS: hypersensitivity to mesna/other thiol compounds ADVERSE EFFECTS: Hypotension THERAPEUTIC USE: Biguanide, Hypoglycemic DOSE: Adult – Diabetes mellitus type 2 a)initial, 500 mg ORALLY twice daily or 850 mg ORALLY once daily b)maintenance, 1 to 2.55 g ORALLY daily in 2-3 divided doses, MAX 2550 mg/day c)(extended release TAB) initial, 500 mg ORALLY once daily d)(extended release TAB) maintenance, 1 to 2 g ORALLY once daily ; MAX 2000 mg/day e)(Fortamet® extended-release) initial, 500 mg to 1 g ORALLY once daily f)(Fortamet® extended-release) maintenance, 1 to 2.5 g ORALLY once daily; MAX 2500 mg/day. Pedia – Diabetes mellitus type 2 a)(immediate release TAB or SOLN) 10-16 yr, initial 500 mg ORALLY twice daily b)(immediate release TAB or SOLN) 10-16 yr, maintenance, titrate in 500 mg increments weekly, MAX 2000 mg/day in divided doses CONTRA-INDICATIONS: hypersensitivity to metformin, iodinated contrast media, intravascular use in radiologic studies; possible acute alteration of renal function resulting in increased risk of lactic acidosis, renal impairment (ie, serum creatinine 1.4 mg/dL or higher in females or 1.5 mg/dL or higher in males), including that caused by cardiovascular collapse, acute myocardial infarction, or septicemia; increased risk of lactic acidosis, metabolic acidosis, acute or chronic, including ketoacidosis; increased risk of lactic acidosis ADVERSE EFFECTS: Lactic acidosis THERAPEUTIC USE: Antimetabolite, Antineoplastic Agent, Antipsoriatic, Antirheumatic, Cytotoxic DOSE: Adult – Non-Hodgkin's lymphoma, Advanced a)(Burkitt's lymphoma, stages I and II) 10 to 25 mg/day ORALLY for 4 to 8 days; (stage III) in combination with other antineoplastic agents b)(lymphosarcoma, stage III) 0.625 to 2.5 mg/kg/day IV/IM in combination with other antineoplastics. Pedia – the safety and effectiveness of methotrexate in pediatric patients has only been established for cancer chemotherapy and polyarticular-course juvenile rheumatoid arthritis. Acute lymphoid leukemia a)induction, 3.3 mg/m(2)/day IV in combination with corticosteroid therapy for 4-6 weeks has been given b)maintenance, 30 mg/m(2)/week administered in 2 divided IM or ORAL doses; 2.5 mg/kg IV every 14 days has also been used CONTRA-INDICATIONS:pregnancy, breastfeeding, known hypersensitivity to methotrexate, patients with psoriasis/rheumatoid arthritis with alcoholism, alcoholic liver disease, or other chronic liver disease, patients with psoriasis/rheumatoid arthritis with preexisting blood dyscrasias or laboratory evidence of immunodeficiency syndromes ADVERSE EFFECTS: Arachnoiditis, With intrathecal administration, Atrophy of liver Cirrhosis of liver, Disorder of lung, Gastrointestinal hemorrhage, Hepatic fibrosis, Hepatic necrosis, Hyperuricemia, Increased liver function test, Inflammatory disease of mucous membrane, Interstitial pneumonia, Acute, chronic, Kidney disease, Liver failure, Myelosuppression, Renal failure, Skin ulcer THERAPEUTIC USE: Diagnostic Agent, Lubricant, Ocular, Surgical Aid, Ocular DOSE: Adult – Gonioscopy; Diagnosis, 2.5% OPHTHALMIC solution used as needed to fill the gonioscopic prism; Operative procedure on anterior chamber of eye – instill a 2% OPHTHALMIC solution into the anterior chamber, as needed, using a 20-gauge or larger cannula; Xerophthalmia – instill 1 drop 0.3% to 1% OPHTHALMIC solution into affected eye(s) 3 to 4 times daily, as needed. Pedia – Xerophthalmia (adolescents) instill 1 drop 0.3% to 1% OPHTHALMIC solution into affected eye(s) 3 to 4 times daily, as needed CONTRA-INDICATIONS: hypersensitivity to hypromellose THERAPEUTIC USE: Alpha-Adrenergic Agonist, Antihypertensive, Cardiovascular Agent DOSE: Adult – Hypertension a)initial, 250 mg ORALLY two-three times daily; may adjust dose at intervals of not less than every 2 days; maintenance, 500-2000 mg ORALLY daily in 2-4 divided doses; MAX dose, 3000 mg daily b)250-500 mg IV every 6 hr; MAX dose, 1000 mg IV every 6 hr. Pedia – Hypertension a)initial, 10 mg/kg ORALLY daily in 2-4 divided doses; MAX dose, 65 mg/kg or 3000 mg daily, whichever is less b)20-40 mg/kg/day IV divided every 6 hr; MAX dose, 65 mg/kg or 3000 mg daily, whichever is less c)(neonates) 2.5-5 mg/kg ORALLY or IV every 8 hr CONTRA-INDICATIONS: current MAOI therapy, hypersensitivity to methyldopa, liver disease (with or without previous association with methyldopa therapy) ADVERSE EFFECTS: Bone marrow depression, Colitis, Congestive heart failure, Decreased liver function, Rarely fatal, Granulocytopenic disorder, Hemolytic anemia, Immune hypersensitivity reaction, Leukopenia, Malignant lymphoma, Neutropenia, Pancreatitis, Parkinsonism, Systemic lupus erythematosus, Thrombocytopenia THERAPEUTIC USE: Antiseptic, Diagnostic Agent, Kidney Function, Toxicology-Antidote Agent DOSE: Adult & Pedia – Drug-induced methemoglobinemia, 1 to 2 mg/kg (0.1 to 0.2 mL/kg of a 1% solution) IV very slowly over several min. CONTRA-INDICATIONS: hypersensitivity to methylene blue, intraspinal injection, methemoglobinemia in cyanide poisoning ADVERSE EFFECTS: Cardiac dysrhythmia, Hemolytic anemia, Malignant hyperthermia, Methemoglobinemia 45 PRODUCT DESCRIPTION Methylergometrine Maleate TRADE NAME Methergin Methylparaben DOSAGE FORM/STRENGTH Injection 0.2mg/mL Powder Methylphenidate Ritalin Tablet 10mg Methylprednisolone Acetate Depo Medrol Injection 40mg Inj. 80mg/2mL Methylprednisolone Sodium Succinate Solu – Medrol Injection 40mg Inj. 125mg/2mL Injection 1gram Metoclopramide Plasil Premosan Primperan Riamide Inj. 10mg/2mL Syrup 5mg/5mL Tablet 10mg Metronidazole Amrizole Anazol Flagyl Flazol Negazole Inj. 500mg Susp. 200mg/5mL Tablet 500mg Vaginal Tab.500mg PRODUCT INFORMATION THERAPEUTIC USE: Ergot Alkaloid, Uterine Stimulant DOSE: Adult – Postpartum hemorrhage, associated with uterine atony or subinvolution, 0.2 mg IM/IV (may be repeated at 2 to 4 h intervals up to 5 doses) then 0.2 mg ORALLY 3 to 4 times a day as needed (MAX duration: 7 days). Pedia – safety and efficacy not established in pediatric patients CONTRA-INDICATIONS: hypersensitivity to methylergonovine products, concomitant use with potent CYP 3A4 inhibitors, pregnancy, toxemia, uncontrolled hypertension ADVERSE EFFECTS: Angina, Hypertensive episode, Myocardial infarction, Seizure THERAPEUTIC USE: Preservative for galenicals in concentrations ranging from 0.05 to 0.25%. When desired to give a strong antiseptic effect, 3 to 5 times the above concentration may be used. DOSE: Topical to pharynx, 3mg in a troche. THERAPEUTIC USE: Amphetamine Related, Central Nervous System Agent & Stimulant DOSE: Adult – individualize dosage according to need and response of patient; Attention deficit hyperactivity disorder a)immediate-release (IR), 10 to 60 mg/day ORALLY divided 2 to 3 times daily, preferably 30 to 45 min before meals b)extended-release, no prior methylphenidate therapy, 18 mg ORALLY once daily in the morning; may adjust dosage at weekly intervals in 18 mg increments; MAX 54 mg/day. Pedia - Attention deficit hyperactivity disorder, (age 6 y and older) immediate-release, 5 mg ORALLY twice daily (before breakfast and lunch); dose adjustments of 5 to 10 mg at weekly intervals; MAX 60 mg/day CONTRA-INDICATIONS: marked agitation, anxiety, and tension; may aggravate symptoms glaucoma, hypersensitivity to methylphenidate or other components of the products, monoamine oxidase inhibitors, such as isocarboxazid, phenelzine, selegiline, tranylcypromine, within 14 days of administration; may cause hypertensive crisis, tics, motor Tourette's syndrome, family history or diagnosis ADVERSE EFFECTS: Contact dermatitis, Decreased body growth, Drug dependence, Lowered convulsive threshold, Psychotic disorder, Tic THERAPEUTIC USE: Adrenal Glucocorticoid, Endocrine-Metabolic Agent, Immune Suppressant DOSE: Adult – Adrenal insufficiency, 40 mg IM every other week; Rheumatoid arthritis a)IM, maintenance, 40-120 mg/wk b)INTRAARTICULAR, 20-80 mg for large joint; 10-40 mg for medium joint; 4-10 mg for small joint, may be repeated every 1-5 weeks or more. Pedia – dosage will be reduced but should be governed more by the severity of the condition and response of the patient than by age or size. Allergic disorder, dosage varies; administer the equivalent of a total daily oral dose as a single IM injection in a 24-h period CONTRA-INDICATIONS: systemic fungal infection, administration of live or live, attenuated vaccine in patients receiving immunosuppressive doses of corticosteroids premature infants, hypersensitivity to methylprednisolone or any component of the product ADVERSE EFFECTS: Congestive heart failure, Drug-induced adrenocortical insufficiency, HPA, Glaucoma, Hyperglycemia, Osteoporosis, Pulmonary tuberculosis, Raised intracranial pressure, Seizure THERAPEUTIC USE: Adrenal Glucocorticoid, Endocrine-Metabolic Agent, Immune Suppressant DOSE: Adult & Pedia – Allergic disorder, initial dose, 10 to 40 mg IV over several minutes, may repeat as IV or IM injections as needed depending on patient's response; for high-dose therapy, 30 mg/kg IV over at least 30 min, may repeat every 4 to 6 h for up to 72 h. CONTRA-INDICATIONS: hypersensitivity to any constituent of the product, premature infants (preparations containing benzyl alcohol), systemic fungal infections, live vaccine or attenuated live vaccine in patients receiving immunosuppressive doses ADVERSE EFFECTS: Cataract, Congestive heart failure, Cushing's syndrome, Glaucoma, Hyperglycemia, Osteoporosis, Primary adrenocortical insufficiency, Pulmonary tuberculosis, Raised intracranial pressure, Seizure THERAPEUTIC USE: Antiemetic, Diagnostic Agent, Dopamine Antagonist, Stimulant, Gastrointestinal DOSE: Adult – Chemotherapy-induced nausea and vomiting; Prophylaxis, 1 to 2 mg/kg/dose IV over 15 min administered 30 min prior to chemotherapy and then every 2 h for 2 doses, then every 3 h for 3 doses. Pedia – Postoperative nausea and vomiting, 0.25 mg/kg/dose IV repeat every 6-8 hrs as needed CONTRA-INDICATIONS: concomitant use of drugs with extrapyramidal adverse effects, gastrointestinal hemorrhage, obstruction (mechanical), or perforation, hypersensitivity to metoclopramide products, pheochromocytoma, seizure disorders ADVERSE EFFECTS: Cardiac dysrhythmia, Reversible, Neuroleptic malignant syndrome THERAPEUTIC USE: Amebicide, Extraintestinal & Intestinal, Antiacne Antibacterial, Antibiotic, Antiprotozoal, Antiulcer, Helicobacter Pylori, Nitroimidazole DOSE: Adult – Abscess, Anaerobic, loading dose, 15 mg/kg IV over 1 h; maintenance dose, 7.5 mg/kg IV every 6 h, starting 6 h after loading dose; MAX 4 g/day; 7.5 mg/kg ORALLY every 6 h; maximum 4 g/day. Pedia – Amebic dysentery, acute, 35-50 mg/kg/day ORALLY in 3 divided doses for 10 days; maximum 750 mg/dose CONTRA-INDICATIONS: hypersensitivity to metronidazole or other nitroimidazole agents, hypersensitivity to parabens (gel formulations), first trimester of pregnancy ADVERSE EFFECTS: Leukopenia, Ototoxicity, Thrombocytopenia 46 PRODUCT DESCRIPTION Miconazole TRADE NAME Daktarin Miconaz Mycoheal DOSAGE THERAPEUTIC USE: Antifungal, Imidazole DOSE: Adult & 12years old – Candidal vulvovaginitis a)200 mg Vaginally at bedtime for 3 days b)100 mg Vaginally at bedtime for 7 days Cream 2% c)1200 mg VAGINALLY once; Tinea, Superficial, apply TOPICALLY to affected areas twice daily. Pedia – Tinea, Superficial, (2 y and older) Vaginal Cream 2% apply TOPICALLY to affected areas twice daily Inj. 2.5mg/0.5mL Inj. 15mg/3mL Midazolam Dormicum Milrinone Primacor Injection 10mg/10mL Mineral Oil/Anhyd. Liquid Lanolin/Petrolatum Refresh PM Eye Ointment Syrup 1mg/mL (30mL, 45mL, 60mL and 75mL) Mirtazapine Remeron Tablet 30mg Misoprostol Cytotec Tablet 200mcg Mitomycin Mitoxantrone HCl Injection 10mg Novantrone PRODUCT INFORMATION FORM/STRENGTH Injection 2mg/mL CONTRA-INDICATIONS:hypersensitivity to miconazole products THERAPEUTIC USE: Anesthetic Adjunct, Benzodiazepine, Short or Intermediate Acting, Hypnotic DOSE: Adult – Amnesia induction – Anxiety – Preoperative sedation a)good risk patients under the age of 60, 0.07 to 0.08 mg/kg IM (approximately 5 mg) 1 h before surgery b)patients with chronic obstructive pulmonary disease, other higher risk surgical patients, patients 60 or more years of age, and patients who have received concomitant narcotics or other CNS depressants, 0.02 to 0.05 mg/kg IM (approximately 2 to 3 mg) 1 h before surgery c)older patients when the anticipated intensity and duration of sedation is less critical, 1 mg IM 1 h before surgery. Pedia – Amnesia induction – Anxiety – Preoperative sedation a)0.1 to 0.15 mg/kg IM, 0.5 mg/kg for more anxious patients. MAX 10 mg b)(6 mos to 5 y of age) initial dose 0.05 to 0.1 mg/kg IV, a total dose of 0.6 mg/kg may be necessary. MAX 6 mg c)(6 to 12 y of age) initial dose 0.025 to 0.05 mg/kg IV, a total dose of 0.4 mg/kg may be necessary. MAX 10 mg d)(12 y of age and older) same dose as adults. MAX 10 mg CONTRA-INDICATIONS: hypersensitivity to midazolam or benzodiazepines, acute narrow-angle glaucoma, untreated open-angle glaucoma, intrathecal or epidural administration (due to the presence of the preservative benzyl alcohol) ADVERSE EFFECTS: Agitation, Apnea, Cardiac arrest, Usually in combinations with CNS depressant drug, Desaturation of blood, Pediatric patients, Hypotensive episode, Involuntary movement, Respiratory arrest, With CNS depressant drugs, Respiratory depression, Respiratory obstruction THERAPEUTIC USE: Inotropic Agent, Vasodilator DOSE: Adult – Congestive heart failure, initial loading dose, 50 mcg/kg IV over 10 min; maintenance, 0.375 to 0.75 mcg/kg/min continuous IV infusion. Pedia – Not FDA approved in children. Decreased cardiac output – Operation on heart, after separation from cardiopulmonary bypass, 50 mcg/kg IV over 15 min followed by 3 mcg/kg/min IV for 30 min; maintenance, 0.5 mcg/kg/min IV CONTRA-INDICATIONS: hypersensitivity to milrinone lactate ADVERSE EFFECTS: Liver function tests abnormal, Thrombocytopenia, Ventricular premature complex THERAPEUTIC USE: Provides strong, soothing nighttime relief for more intense dry, irritated eyes. It is a preservative free lubricant eye ointment that is ideal for use at bedtime. THERAPEUTIC USE: Antidepressant, Tetracyclic DOSE: Adult – Major depressive disorder, 15 mg/day ORALLY at bedtime; may increase in dose every 1-2 weeks to a max dose of 45 mg/day. Pedia - safety and effectiveness in pediatric patients have not been established CONTRA-INDICATIONS: hypersensitivity to mirtazapine ADVERSE EFFECTS: Agranulocytosis, Depression, worsening, Mania, Neutropenia, Seizure, Suicidal thoughts, Suicide THERAPEUTIC USE: Antiulcer, Protectant, Endocrine-Metabolic Agent, Prostaglandin DOSE: Adult – Abortion, 800 mcg VAGINALLY times one dose; Cervical ripening procedure – Induction of labor a)25 mcg VAGINALLY every 3 to 4 hours (maximum dose, 400 mcg) b)initial, 100 mcg ORALLY every 4 hours (maximum of 5 doses) c)initial, 50 mcg ORALLY every 4 hours for 2 doses, then 100 mcg every 4 hours until membrane rupture (maximum of 5 doses). Pedia – safety and efficacy have not been established in pediatric patients CONTRA-INDICATIONS: hypersensitivity to misoprostol or prostaglandins, pregnancy ADVERSE EFFECTS: Anemia, Cardiac dysrhythmia THERAPEUTIC USE: Antibiotic, Antineoplastic Agent DOSE: Adult – Carcinoma of pancreas, Disseminated adenocarcinoma, in combination with other agents, 20 mg/m(2)/dose IV every 6 to 8 weeks CONTRA-INDICATIONS: coagulation disorders or increase bleeding tendency, hypersensitivity to mitomycin, no dosages should be repeated unless leukocyte count has returned to 4000/m(3) and platelet count to 100,000/m(3), renal impairment: do not give to patients with a SCr>1.7 mg/dL ADVERSE EFFECTS: Hemolytic uremic syndrome, Myelosuppression,Nephrotoxicity THERAPEUTIC USE: Antineoplastic Agent DOSE: Adult - Acute myeloid leukemia, In combination with other approved agents a)induction, 12 mg/m(2) IV daily on days 1-3, in combination with cytarabine 100 mg/m(2) daily as continuous IV infusion on days 1-7 b)re-induction, if incomplete response to first induction, may give second induction dose, 12 mg/m(2) IV daily for 2 days in combination with cytarabine 100 mg/m(2) daily as continuous IV infusion on days 1-5 c)consolidation, 12 mg/m(2) IV daily on days 1 and 2, in combination with cytarabine 100 mg/m(2) daily as continuous IV infusion 47 PRODUCT DESCRIPTION TRADE NAME DOSAGE FORM/STRENGTH Mivacurium Chloride Mivacron Injection 2mg/mL Monobasic Na Phosphate/ Dibasic Na Phosphate Fleet Enema Enema Adult Enema Pedia Morphine Sulphate MST Inj. 10mg/mL Tablet 30mg Tablet 60mg Moxifloxacin HCl Avalox Vigamox Eye Drops 0.5% Injection 400mg Tablet 400mg Multivitamin Mixavit Injection Adult Injection Pedia Syrup Tablet Multivitamins/Iron Polyvisol Drops PRODUCT INFORMATION on days 1-5; the first course is usually started 6 wk after final induction dose and the second, 4 weeks after the first . Pedia – safety and effectiveness not established in pediatric patients. Solid tumor configuration, 5-8 mg/m(2)/week IV; alternative dosing regimen 18-20 mg/m(2) IV every 3-4 weeks CONTRA-INDICATIONS: hypersensitivity to mitoxantrone products ADVERSE EFFECTS: Acute myeloid leukemia, Secondary, Cardiotoxicity, Hepatotoxicity, Myelodysplasia of the spinal cord, Myelosuppression THERAPEUTIC USE: Musculoskeletal Agent, Neuromuscular Blocker, Non-Depolarizing DOSE: Adult – dosage should be individualized, Induction of neuromuscular blockade, During surgery as an adjunct to general anesthesia and to facilitate tracheal intubation and to provide skeletal muscle relaxation during surgery or mechanical ventilation a)initial, for intubation, 0.15 mg/kg IV bolus over 5-15 sec or 0.2 mg/kg IV bolus over 30 sec OR 0.25 mg/kg IV bolus in divided doses (0.15 mg/kg followed in 30 sec by 0.1 mg/kg) b)maintenance, 0.1 mg/kg IV bolus 15-25 min after initial dose c)maintenance, 9-10 mcg/kg/min continuous IV infusion after initial dose, upon early evidence of spontaneous recovery d)maintenance, 4 mcg/kg/min continuous IV infusion if initiated simultaneously with administration of initial dose. Pedia – dosage should be individualized, Induction of neuromuscular blockade, During surgery as an adjunct to general anesthesia and to facilitate tracheal intubation and to provide skeletal muscle relaxation during surgery or mechanical ventilation a)(age 2-12 yr) initial, for intubation, 0.2 mg/kg IV bolus over 5-15 seconds b)(age 2-12 yr) maintenance, 0.1 mg/kg IV bolus c)(age 2-12 yr) maintenance, 14 mcg/kg/min (range 5-31 mcg/kg/min) continuous IV infusion after initial dose, upon early evidence of spontaneous recovery CONTRA-INDICATIONS: hypersensitivity to mivacurium products/benzylisoquinolinium, hypersensitivity to benzyl alcohol (multi-dose vials) ADVERSE EFFECTS: Bradyarrhythmia, Cardiac dysrhythmia, Hypotension, Prolonged neuromuscular block, Tachyarrhythmia THERAPEUTIC USE: Colorectal Agent, Laxative, Hyperosmotic, Phosphate Supplement DOSE: Adult – one bottle (118mL delivered dose) or as directed by physician. Pedia – one bottle (59mL delivered dose) or as directed by physician. Do not use on children under 2years of age unless directed by a physician. CONTRA-INDICATIONS: acute phosphate nephropathy, biopsy-proven, allergy or hypersensitivity to sodium phosphate salts or any product component ADVERSE EFFECTS: Phosphate nephropathy, acute, Prolonged QT interval, Renal failure, Tonic-clonic seizure THERAPEUTIC USE: Analgesic, Analgesic Combination, Opioid, Anesthetic Adjunct Opioid DOSE: Adult – Acute myocardial infarction – Pain, 2 to 10 mg IV administered slowly over 4 to 5 min, a strength of 2.5 to 15 mg may be diluted in 4 to 5 mL of sterile water for injection; Pain (Moderate to Severe), 0.1 to 0.2 mg/kg SUBQ every 4 h, MAX 15 mg/dose; 0.05 to 0.1 mg/kg IV, MAX 10 mg/dose; (neonates) 0.1 mg/kg IV/SUBQ every 4 to 6 h CONTRA-INDICATIONS: asthma, severe or acute, hypercarbia, hypersensitivity to morphine, morphine salts, or to any component of the products, paralytic ileus, known or suspected, respiratory depression; in the absence of resuscitative equipment or in unmonitored settings, upper airway obstruction ADVERSE EFFECTS: Anaphylaxis, Cardiac arrest, Dyspnea, Finding of intracranial pressure, Myoclonus, Orthostatic hypotension, Other specified circulatory disorder, circulatory depression, Respiratory depression, Shock, Syncope THERAPEUTIC USE: Antibiotic, Fluoroquinolone DOSE: Adult – Acute exacerbation of chronic obstructive pulmonary disease, 400 mg IV or ORALLY every 24 hr for 5 days; Bacterial conjunctivitis, (0.5% ophthalmic solution) 1 drop to affected eye(s) 3 times a day for 7 days; Bacterial sinusitis, acute 400 mg IV or ORALLY every 24 hr for 10 days. Pedia – safety and efficacy of oral and parenteral moxifloxacin in children less than 18 years of age have not been established; Bacterial conjunctivitis, (0.5% ophthalmic solution; 1 year of age and older) 1 drop to affected eye(s) 3 times a day for 7 days CONTRA-INDICATIONS: hypersensitivity to moxifloxacin hydrochloride or quinolone antibiotics ADVERSE EFFECTS: Acute renal failure, Anaphylactoid reaction, Aplastic anemia, Extrinsic allergic alveolitis, Hemolytic anemia, Hepatic necrosis, Hepatitis, Immune hypersensitivity reaction, Jaundice, Liver failure, Pancytopenia, Peripheral neuropathy, Prolonged QT interval, Serum sickness due to drug, Stevens-Johnson syndrome, Thrombocytopenia, Torsades de pointes, Toxic epidermal necrolysis, Traumatic or non-traumatic rupture of tendon THERAPEUTIC USE: To avoid and correct inadequate vitamin intake which may result from an imbalanced or special diet, impaired absorption, anorexia, slimming or therapy with drugs acting as vitamin antagonists. DOSE: Adult – 1 tablet three times a day. Pedia – above 4years, 1teaspoonful once daily; 2 to 4 years, 1/2 teaspoonful once daily. All doses above or as directed by the physician. THERAPEUTIC USE: To avoid and correct inadequate vitamin intake which may result from an imbalanced or special diet, impaired absorption, anorexia, slimming or therapy with drugs acting as vitamin antagonists. DOSE: DROPS 0.3 – 0.6mL (1/2 – full dropper) daily or as directed by the physician. 48 PRODUCT DESCRIPTION Mupirocin Naloxone Hydrochloride TRADE NAME Bactroban Narcan Naxone DOSAGE FORM/STRENGTH Nasal Ointment 2% Ointment 2% Injection 0.4mg/mL Neomycin/Polymyxin /Hydrocortisone Otosporin Ear Drops Neomycin/Polymyxin /Dexamethasone Maxitrol Eye Drops Eye Ointment Neostigmine Bromide Prostigmin Tablet 15mg Neostigmine Methylsulphate Prostigmin Inj. 0.5mg/mL Inj. 2.5mg/mL Niclosamide Yomesan Tablet 500mg PRODUCT INFORMATION THERAPEUTIC USE: Antibacterial, Antibiotic DOSE: Adult – Impetigo, apply 2% ointment TOPICALLY 3 times per day for 3-5 days, reevaluate if no response; Methicillin resistant Staphylococcus aureus infection, Colonization, intranasal ointment, apply one-half of single-use 1 g tube into each nostril TOPICALLY twice daily for 5 days. Pedia – Impetigo (2 months to 15 years of age) apply 2% ointment TOPICALLY 3 times a day for 3-5 days; reevaluate if no response; Methicillin resistant Staphylococcus aureus infection, Colonization (12 years of age and older) intranasal ointment, apply one-half of single-use 1 g tube into each nostril TOPICALLY twice daily for 5 days CONTRA-INDICATIONS: hypersensitivity to mupirocin or any component of the product THERAPEUTIC USE: Opioid Antagonist, Toxicology-Antidote Agent DOSE: Adult – Methadone overdose, 0.4 to 2 mg IV, repeat every 2 to 3 min as needed; if no response after 10 mg, reconsider diagnosis of opioid toxicity; may administer IM or SC if IV access not available. Pedia – Methadone overdose, a)(neonates) 0.01 mg/kg IV/IM/SC every 2 to 3 min to desired degree of reversal b)0.01 mg/kg IV, then 0.1 mg/kg if needed; may give IM/SC in divided doses if IV route not available c)age less than 5 yr OR weight 20 kg or less, 0.1 mg/kg IV/IO/ET; age 5 yr and older OR weight greater than 20 kg, 2 mg CONTRA-INDICATIONS: hypersensitivity to naloxone ADVERSE EFFECTS: Cardiac dysrhythmia, Hepatotoxicity, Hypertension, Hypotension, Opioid withdrawal, Pulmonary edema, Ventricular fibrillation THERAPEUTIC USE: Aminoglycoside/Corticosteroid Combination, Anti-Infective/Anti-Inflammatory Combination DOSE: Adult – Infection of external auditory canal, instill 4 drops OTICALLY in affected ear(s) 3 to 4 times a day; MAX duration 10 days. Pedia – Ophthalmic suspension not FDA-approved in children, Infection of external auditory canal, 2 y and older, instill 3 drops OTICALLY in affected ear(s) 3 to 4 times a day; MAX duration 10 days CONTRA-INDICATIONS: Use in eyes or in external ear canal if eardrum is perforated, Use on tuberculous, fungal, or viral lesions, Hypersensitivity to any of its components, Viral diseases of the cornea and conjunctiva, Mycobacterial infection, Fungal infections of the ocular structures, Cutaneous viral infections such as herpes simplex virus or varicella zoster virus THERAPEUTIC USE: Indicated in ocular inflammation when concurrent use of an antimicrobial is judged necessary. DOSE: Eye Drops – 1 to 2 drops topically in the conjunctival sac. In severe disease, drops may be used hourly, being tapered to discontinuation as the inflammation subsides; Eye Ointment – apply a small amount into the conjunctival sac up to 3 or 4 times daily or may be used adjunctively with drops at bedtime. CONTRA-INDICATIONS: Epithelial herpes simplex keratitis, vaccinia, varicella and many other viral diseases of the cornea & conjunctiva. Hypersensitivity to a component of the medication. THERAPEUTIC USE: Central Nervous System Agent, Cholinesterase Inhibitor, Immunological Agent, Nondepolarizing Muscle Relaxant Antagonist DOSE: Adult – Myasthenia gravis, 15 to 375 mg ORALLY daily; average dose, 150 mg ORALLY over 24 h; individualize interval between doses and adjust as needed. Pedia – safety and effectiveness not established in children CONTRA-INDICATIONS: hypersensitivity to neostigmine or bromides, intestinal or urinary obstruction, peritonitis ADVERSE EFFECTS: Anaphylaxis, Bronchospasm, Cardiac dysrhythmia, Respiratory arrest, Respiratory depression, Seizure THERAPEUTIC USE: Central Nervous System Agent, Cholinesterase Inhibitor, Immunological Agent, Nondepolarizing Muscle Relaxant Antagonist DOSE: Adult – Abdominal distension – Postoperative complication; Treatment and prophylaxis, 0.25 mg SUBQ or IM as soon as possible after operation, repeat every 4 to 6 h for 2 to 3 days; treatment, 0.5 mg SUBQ or IM as required THERAPEUTIC USE: Anthelmintic DOSE: Adult – usual oral dose 2 grams (4 tablets) as a single dose daily for 7 days. Hymenolepis nana does not need an intermediate host before infecting humans, thus both the adult worm and the larva exist in the intestine. Therapy must be continued for 7 days to ensure the infestation is eradicated. Pedia – In children weighing more than 34 kg (75 pounds) the recommended dose for taenia saginata, taenia solium, and diphyllobothrium latum is 3 tablets (1.5 grams) chewed thoroughly as a single dose CONTRA-INDICATIONS: Hypersensitivity to niclosamide 49 PRODUCT DESCRIPTION Nifedipine Nitrazepam Nitrofurantoin Noradrenaline Acid Tartrate TRADE NAME Adalat Adalat Retard Coracten Epilat Epilat Retard Nifecard Nifecard Retard Mogadon Furadantin Macrodantin Levophed Mikostat Nystatin Mycostatin Rianest Octreotide Sandostatin DOSAGE FORM/STRENGTH Capsule 10mg Tablet 20mg Tablet 5mg Susp. 25mg/5mL Tablet 100mg Injection 1:1000 Ointment 100,000u/g Susp. 100,000u/mL Inj. 0.1mg/mL Inj. 0.2mg/mL PRODUCT INFORMATION THERAPEUTIC USE: Antianginal, Antihypertensive, Antimigraine Calcium Channel Blocker, Cardiovascular Agent, Dihydropyridine DOSE: Adult – Hypertension (extended-release tablets) initial 30 to 60 mg ORALLY once daily; maintenance, 30 to 90 mg ORALLY once daily, MAX 120 mg/day. Pedia – not FDA approved for pediatric patients. Hypertrophic cardiomyopathy 0.6-0.9 mg/kg/24hr ORALLY divided into 3-4 doses CONTRA-INDICATIONS: hypersensitivity to nifedipine or other calcium channel antagonists ADVERSE EFFECTS: Angina, Increased, Myocardial infarction THERAPEUTIC USE: Anticonvulsant, Benzodiazepine, Short or Intermediate Acting, Sedative-Hypnotic DOSE: Adult - Oral route a)Doses most commonly used to treat INSOMNIA in clinical studies have been nitrazepam 5 to 10 mg at bedtime; however, extreme hangover effects and side effects limit this dosage. b)Nitrazepam 5 milligrams at bedtime is considered an effective dose for relieving anxiety induced sleep disorders before DENTAL PROCEDURES.Pedia – has been used extensively in the treatment of EPILEPSY in children. Doses used in most clinical trials have ranged from 1 to 6 milligrams daily initially, with doses increasing up to 60 mg daily CONTRA-INDICATIONS: Hypersensitivity to nitrazepam THERAPEUTIC USE: Antibiotic, Nitrofuran DOSE: Adult – Urinary tract infectious disease, 50 to 100 mg ORALLY 4 times a day for 1 week or at least 3 days after urine is sterile. Pedia – contraindicated in neonates below the age of 1 month. Urinary tract infectious disease (1 mo and older) 5 to 7 mg/kg/day in 4 divided doses for 1 week or at least 3 days after urine is sterile CONTRA-INDICATIONS: Known hypersensitivity to nitrofurantoin, Creatinine clearance less than 60 mL/min, Pregnancy at term, Infants less than 1 month of age, Perinephric abscess or pyelonephritis, Prostate infection in elderly men ADVERSE EFFECTS: Cholestatic jaundice syndrome, Hemolytic anemia, Hepatic necrosis, Hepatitis, Immune hypersensitivity reaction, Interstitial lung disease, Neuropathy, Pulmonary fibrosis, Pulmonary toxicity THERAPEUTIC USE: Adrenergic, Sympathomimetic, Vasopressor DOSE: Adult – Hypotension, acute a)initial, 8 to 12 mcg/min IV and observe response; adjust rate of flow to establish a low normal BP (systolic, 80 to 100 mmHg) b)maintenance, 2 to 4 mcg/min IV; doses up to 68 mg/day may be needed. Pedia – safety and efficacy not established in pediatric patients; Hypotension, acute initial 0.1 mcg/kg/min IV, titrate to desired effect; maintenance 0.05 to 0.3 mcg/kg/min, MAX 6 mcg/min CONTRA-INDICATIONS: hypotension due to blood volume deficit ADVERSE EFFECTS: Cardiac arrest, Cardiac dysrhythmia THERAPEUTIC USE: Antifungal, Polyene DOSE: Adult – Candidal vulvovaginitis 1 tablet (100,000 units) intravaginally daily for 2 wks; Candidiasis of skin, Cutaneous and mucocutaneous infections; ointment or cream, apply liberally to affected areas topically twice daily until healing complete. Pedia Candidiasis of skin, Cutaneous and mucocutaneous infections, ointment or cream, apply liberally to affected areas topically twice daily until healing complete; powder, apply to candidal lesions topically 2 to 3 times daily until healing complete; for fungal infections of the feet, footwear should be dusted as well CONTRA-INDICATIONS: hypersensitivity to nystatin products THERAPEUTIC USE: Endocrine-Metabolic Agent, Somatostatin (class) DOSE: Adult – Acromegaly, Inadequate response to or ineligible for surgery, radiation, or bromocriptine mesylate a)initial, 50 mcg SC/IV 3 times daily b)maintenance, 100-500 mcg SC/IV 3 times daily c)initial, 20 mg IM intragluteally at 4-week intervals for 3 months d)after initial 3 months, continue 20 mg IM intragluteally every 4 weeks if GH is </= 2.5 ng/mL, IGF-I is normal, and clinical symptoms have improved e)after initial 3 months, increase to 30 mg IM intragluteally every 4 weeks if GH is > 2.5 ng/mL, IGF-I is elevated, and/or clinical symptoms uncontrolled f)after initial 3 months, decrease to 10 mg IM intragluteally every 4 weeks if GH is </= 1 ng/mL, IGF-I is normal, and clinical symptoms are controlled g)increase dose to 40 mg IM intragluteally every 4 weeks in patients whose GH, IGF-1, and symptoms are not adequately controlled at 30 mg. Pedia – Safety and efficacy in children not established CONTRA-INDICATIONS: sensitivity to octreotide or any of its components ADVERSE EFFECTS: Cardiac dysrhythmia, Congestive heart failure, Worsening, Sinus bradycardia 50 PRODUCT DESCRIPTION TRADE NAME DOSAGE FORM/STRENGTH Ofloxacin Eylox Oflox Oxacin Eye Drops Ofloxacin/Prednisolone /Tetrahydrozoline Loxtra Eye Drops Omeprazole Hyposec Losec Omeral Risek Mups Tab 10mg Ondansetron HCl Dihydrate Zofran Inj. 4mg/2mL Inj. 8mg/4mL Tablet 4mg Tablet 8mg Oxybuprocaine Benoxinate Oxybutynin Ditropan Tablet 5mg Oxytocin Syntocinon Inj. 5units/mL Inj. 10units/mL Eye Minims 0.4% PRODUCT INFORMATION THERAPEUTIC USE: Antibacterial, Antibiotic, Fluoroquinolone DOSE: Adult – Bacterial conjunctivitis (ophthalmic) day 1 and 2, instill 1 to 2 drops in affected eye(s) every 2 to 4 h; day 3 to 7, instill 1 to 2 drops 4 times daily. Pedia – safety and efficacy in children less than 18 years of age have not been established (oral) Bacterial conjunctivitis, (ophthalmic; 1 y of age and older) day 1 and 2, instill 1 to 2 drops in affected eye(s) every 2 to 4 h; day 3 to 7, instill 1 to 2 drops 4 times daily THERAPEUTIC USE: For corticosteroid responsive inflammatory conditions of the conjunctiva, cornea and anterior segment of the eye where bacterial infection or a risk of bacterial infection exists. DOSE: Apply 1 to 2 drops three to four times daily in the affected eye(s). CONTRA-INDICATIONS: Epithelial herpes simplex keratitis, vaccinia, varicella and many other viral diseases of the cornea and conjunctiva, tuberculosis of the eye, fungal diseases of the ocular structures, hypersensitivity to any ingredient of the medication. ADVERSE EFFECTS: Local irritation, including photophobia, blurred vision, dizziness, numbness, nausea and headache THERAPEUTIC USE: Antiulcer, Gastrointestinal Agent, Proton Pump Inhibitor DOSE: Adult – Duodenal ulcer disease, Dual therapy; Adjunct – Helicobacter pylori gastrointestinal tract infection, Dual therapy. Pedia – Safety not established in pediatric patients. Erosive esophagitis, To maintain healing; Prophylaxis – Gastroesophageal reflux disease, To maintain healing; Prophylaxis a)2 y and older, less than 20 kg, 10 mg ORALLY daily b)2 y and older, 20 kg or greater, 20 mg ORALLY daily CONTRA-INDICATIONS: Hypersensitivity to omeprazole or to any of its components ADVERSE EFFECTS: Hepatotoxicity, Hip fracture, Interstitial nephritis, Pancreatitis, Rhabdomyolysis THERAPEUTIC USE: Antiemetic, Serotonin Receptor Antagonist, 5-HT3 DOSE: Adult – Chemotherapy-induced nausea and vomiting, Initial and repeat courses of moderately emetogenic chemotherapy; Prophylaxis, 8 mg dissolved orally on tongue 30 min prior to chemotherapy and repeated in 8 hours, then 8 mg every 12 hours for 1 to 2 days post chemotherapy. Pedia – Chemotherapy-induced nausea and vomiting, Initial and repeat courses of moderately emetogenic chemotherapy; Prophylaxis a)(IV) 6 months and older, 0.15 mg/kg IV 30 min prior to chemotherapy, repeat 4 and 8 hours after the first dose b)(oral) 4-11 y, 4 mg orally 30 min prior to chemotherapy, repeated 4 and 8 hours after the first dose, then every 8 hours for 1-2 days post chemo c)(oral) 12 y and older, 8 mg ORALLY 30 min prior to chemo and repeat in 8 hours, then 8 mg every 12 hours for 1 to 2 days post chemotherapy CONTRA-INDICATIONS: hypersensitivity to ondansetron ADVERSE EFFECTS: Bronchospasm, Cardiac dysrhythmia THERAPEUTIC USE: Amino Ester, Anesthetic, Local DOSE: Adult – Benoxinate should not be used on infected areas. For anesthesia during short ophthalmologic procedures, a 0.4% benoxinate solution is recommended. Dosages are one to two drops for tonometry, two drops for the fitting of contact lenses and three to six drops for the removal of foreign bodies from the corneal epithelium or minor surgery. The benoxinate drops should be instilled into the conjunctival sac at 30- to 90-second intervals. Pedia – Two drops of a 0.4% benoxinate solution demonstrated excellent efficacy in peri-operative analgesia in a randomized study of 40 children aged 3 to 8 years undergoing strabismus surgery. CONTRA-INDICATIONS: Known hypersensitivity to benoxinate or other anesthetics of the ester type THERAPEUTIC USE: Antimuscarinic, Urinary Antispasmodic DOSE: Adult – Bladder muscle dysfunction - overactive, With symptoms of urge urinary incontinence, urgency, and frequency, extended release, 5 mg or 10 mg once daily at same time each day, dose may be increased in 5 mg increments at weekly intervals to MAX 30 mg daily. Pedia – Bladder muscle dysfunction - overactive, With symptoms of urge urinary incontinence, urgency, and frequency, (age 6 y and older) extended release, 5 mg orally once per day at same time each day; dose may be increased in 5-mg increments to a maximum of 20 mg/day CONTRA-INDICATIONS: gastric retention, glaucoma, narrow-angle (uncontrolled), hypersensitivity to oxybutynin, urinary retention THERAPEUTIC USE: Diagnostic Agent, Fetal Heart Rate Distress, Endocrine-Metabolic Agent, Pituitary Hormone, Posterior, Uterine Stimulant DOSE: Adult – Induction of labor, Medically indicated, initial, 0.5 to 1 milliunit/min IV (3 to 6 mL/h of a 10 units/1000 mL dilute oxytocin solution); gradually increase dose in increments of 1 to 2 milliunits/min every 30 to 60 min until desired contraction pattern has been established; once desired frequency of contractions has been reached and labor progressed to 5 to 6 cm dilation, the dose may be reduced by similar increments CONTRA-INDICATIONS: fetal distress where delivery is not imminent, hypersensitivity to the drug, obstetrical emergencies, significant cephalopelvic disproportion, unfavorable fetal positions or presentations, where adequate uterine activity fails to achieve satisfactory progress, where the uterus is already hyperactive or hypertonic, where vaginal delivery is contraindicated ADVERSE EFFECTS: Afibrinogenemia, Fatal, in mother, Anaphylaxis, Mother, Brain damage, Permanent, Cardiac dysrhythmia, Mother and fetus, Central nervous system deficit, Permanent, Coma, Mother, Convulsions in the newborn, Death, Mother, Fetal bradycardia, Hypertensive episode, Mother, Low apgar score, At 5 minutes, Neonatal jaundice, Pelvic hematoma, Mother, Postpartum hemorrhage, 51 PRODUCT DESCRIPTION TRADE NAME DOSAGE FORM/STRENGTH Paclitaxel Taxol Inj. 30mg/5mL Inj. 150mg/25mL Pancuronium Bromide Pavulon Injection 4mg/2mL Pantoprazole Pantozol Proton Papaverine HCl Vasorin Paracetamol Adol Fevadol Panadol Tempra Tylenol Tylenol Forte Paracetamol/Caffeine /Codeine Paracetamol/Orphenadrine Citrate Injection 40mg Tablet 40mg Injection 0.06g/2mL Drops 100mg/mL Supp.125mg Supp. 250mg Supp. 500mg Syrup 120mg/5mL Tablet 500mg Fevadol Plus Panadol Extra Myogesic Tablet Tablet PRODUCT INFORMATION Mother, Retinal hemorrhage, Neonatal, Rupture of uterus, Mother, Subarachnoid hemorrhage, Mother, Ventricular premature beats, Mother and fetus, Water intoxication syndrome THERAPEUTIC USE: Antineoplastic Agent, Mitotic Inhibitor DOSE: Adult – Breast cancer, Adjuvant, node-positive administered sequentially to the standard doxorubicin-containing regimen, 175 mg/m(2) IV over 3 hr every 3 wk for four courses given sequential to doxorubicin-containing combination chemotherapy. Pedia – safety and efficacy not established in pediatric patients CONTRA-INDICATIONS: hypersensitivity to taxol, Cremophor EL, neutropenia less than 1,500 cells/mm(3)-solid tumors, neutrophil count <1,000 cells/mm(3)-Kaposi's sarcoma ADVERSE EFFECTS: Anaphylaxis, Coronary artery stent thrombosis, Hypotension, Immune hypersensitivity reaction, Myelosuppression, Neutropenic disorder THERAPEUTIC USE: Musculoskeletal Agent, Neuromuscular Blocker, Non-Depolarizing DOSE: Adult – dosage must be individualized. General anesthesia; Adjunct, initial, 0.04 to 0.1 mg/kg IV (endotracheal intubation, 0.06 to 0.1 mg/kg IV); later incremental doses starting at 0.01 mg/kg may be used. Pedia – dosage must be individualized. General anesthesia; Adjunct a)(all ages except neonates) initial, 0.04 to 0.1 mg/kg IV (endotracheal intubation, 0.06 to 0.1 mg/kg IV); later incremental doses starting at 0.01 mg/kg may be used b)(neonates) administer a test dose of 0.02 mg/kg IV to measure responsiveness CONTRA-INDICATIONS: hypersensitivity to pancuronium or bromide products ADVERSE EFFECTS: Apnea, Bronchospasm, Hypertension, Prolonged neuromuscular block, Respiratory failure, Tachyarrhythmia THERAPEUTIC USE: Antiulcer, Gastrointestinal Agent, Proton Pump Inhibitor DOSE: Adult – Duodenal ulcer disease, 40-80 mg ORALLY once daily for 4-8 weeks; Zollinger-Ellison syndrome, 80 mg IV infusion every 12 hours, can increase to every 8 hours; MAX 240 mg/day; 40 mg ORALLY twice daily; MAX 240 mg daily. Pedia – Safety and efficacy not established in children CONTRA-INDICATIONS: hypersensitivity to pantoprazole products ADVERSE EFFECTS: Hip fracture, Hyperglycemia, Rhabdomyolysis, Stevens-Johnson syndrome THERAPEUTIC USE: Peripheral Vasodilator DOSE: Adult – Erectile dysfunction a)initial, 30 mg INTRACAVERNOSAL over 1 to 2 min; may increase to 60 mg according to response b)(combination therapy) 30 mg papaverine / 0.5 to 1 mg phentolamine INTRACAVERNOSAL c)do not use more than 3 times weekly or 2 days in succession. Pedia – not FDA approved in children CONTRA-INDICATIONS: complete atrioventricular block, hypersensitivity to papaverine ADVERSE EFFECTS: Acidosis, Hepatotoxicity, Priapism, Raised intracranial pressure THERAPEUTIC USE: Acetaminophen Combination, Analgesic, Antipyretic DOSE: Adult – Fever a)650 to 1000 mg ORALLY every 4 h as needed, maximum 4 g/day b)650 mg RECTALLY every 4 to 6 h; maximum 6 suppositories/24 h. Pedia – Fever a)10 to 15 mg/kg/dose ORALLY every 4 to 6 h, maximum 5 doses/day b)age 6 to 12 y, 325 mg ORALLY every 4 to 6 h, maximum 2.6 g/24 h c)age 3 to 6 y, 120 to 125 mg RECTALLY every 4 to 6 h; maximum 720 mg/24 h d)age 1 to 3 y, 80 mg RECTALLY every 4 h e)age 3 to 11 months, 80 mg RECTALLY every 6 h CONTRA-INDICATIONS: hypersensitivity to acetaminophen ADVERSE EFFECTS: Gastrointestinal hemorrhage, Hepatotoxicity, Nephrotoxicity, Pneumonitis THERAPEUTIC USE: Analgesic, Opioid/Acetaminophen Combination DOSE: Adult – Pain, Mild to moderately severe a)acetaminophen 300 to 1000 mg (MAX 4000 mg/day) / codeine 15 to 60 mg (MAX 360 mg/day) ORALLY every 4 hours as needed b)15 mL (1 tbsp) ORALLY every 4 hours as needed. Pedia – safety not established in children under 3 years of age. Pain, Mild to moderately severe a)(3-6 yrs) 5 mL ORALLY 3 to 4 times a day b)(7-12 yrs) 10 mL ORALLY 3 to 4 times a day c)for TABS, dose of codeine phosphate is 0.5 mg/kg CONTRA-INDICATIONS: hypersensitivity to acetaminophen/codeine products ADVERSE EFFECTS: Respiratory depression, At higher doses THERAPEUTIC USE: Antimuscarinic, Skeletal Muscle Relaxant, Centrally Acting DOSE: Adult – Musculoskeletal pain, 100 mg ORALLY twice daily in morning and evening; 60 mg IV/IM, may be repeated every 12 hr; switch to oral form for maintenance. Pedia – safety and effectiveness have not been established for pediatric patients CONTRA-INDICATIONS: cardiospasm (megaesophagus), glaucoma, hypersensitivity to orphenadrine, myasthenia gravis, prostatic hypertrophy or obstruction of bladder neck, pyloric or duodenal obstruction, stenosing peptic ulcer ADVERSE EFFECTS: Anaphylaxis, With IM injection, Palpitations, Tachyarrhythmia 52 PRODUCT DESCRIPTION TRADE NAME DOSAGE FORM/STRENGTH Paraffin Soft White Jelly Vaseline Gel Peginterferon Alpha 2B Peg – Intron Injection Pentazocine Sosegon Injection 30mg/mL Pentoxifylline Trental Inj. 100mg/5mL Tablet 400mg Pethidine HCl Demerol Meperidine Inj. 50mg/mL Inj. 100mg/2mL Phenazone/Benzocaine /Glycerol Auralgan Ear Drops Gardenal Inj. 40mg/mL Inj. 130mg/mL Inj. 200mg/mL Syrup 10mg/mL (50mL & 100mL) Tablet 15mg Tablet 100mg Phenobarbitone Phenol Injection 6% Phenoxybenzamine HCl Dibenylin Capsule 10mg Phentolamine Mesylate Regitine Injection PRODUCT INFORMATION THERAPEUTIC USE: As an emollient in the management of skin disorders. It is not readily absorbed by the skin. Sterile dressings containing soft paraffin are used for wound dressing. THERAPEUTIC USE: Indicated for the treatment of chronic hepatitis C in adults. DOSE: Recommended dose is 0.5mcg/Kg administered SC once weekly for one year. CONTRA-INDICATIONS: Hypersensitivity to the active substance or to any interferon or to any of the excipients, pregnancy, men whose female partners are pregnant, autoimmune disease, decompensated liver disease. ADVERSE EFFECTS: Headache, myalgia, pain/inflammation at injection site, fatigue, rigors, fever, depression, arthralgia, nausea, alopecia, musculoskeletal pain, irritability, influenza like symptoms, insomnia, diarrhea, abdominal pain, asthenia, pharyngitis, weight decrease, anorexia, anxiety, dizziness, pruritus. THERAPEUTIC USE: Analgesic, Anesthetic Adjunct, Opioid Agonist/Antagonist DOSE: Adult – Anesthesia; Adjunct, 30 mg IV, IM or SC every 3 to 4 hr as needed, MAX 360 mg/day; doses above 30 mg IV or 60 mg IM, SC are not recommended. Pedia – Safety and effectiveness in children less than 12 yr of age not established. Anesthesia; Adjunct, single 0.5 mg/kg IM dose CONTRA-INDICATIONS: hypersensitivity to pentazocine ADVERSE EFFECTS: Dyspnea, Hallucinations, Injection site necrosis, Physical addiction, Respiratory depression, Toxic epidermal necrolysis THERAPEUTIC USE: Hemorheologic, Methylxanthine DOSE: Adult – Intermittent claudication, 400 mg ORALLY three times a day with meals; Stasis ulcer – 800 mg ORALLY three times a day; Vascular disorder of inner ear, 1600 mg/day ORALLY in 2-4 divided doses. Pedia – safety and effectiveness in pediatric patients have not been established. CONTRA-INDICATIONS: hypersensitivity to pentoxifylline or methylxanthines, recent cerebral hemorrhage, recent retinal hemorrhage ADVERSE EFFECTS: Angina, Aplastic anemia, Cardiac dysrhythmia, Confusion, Depression, Edema, Hepatitis, Hypotension, Increased liver function test, Jaundice, Leukopenia, Seizure, Thrombocytopenia THERAPEUTIC USE: Analgesic, Anesthetic Adjunct, Opioid DOSE: Adult – Obstetric pain, 50 to 100 mg IM/subQ every 1 to 3 hr as needed CONTRA-INDICATIONS: hypersensitivity to meperidine, recent or concomitant MAOI ADVERSE EFFECTS: Cardiac arrest, Disorder of cardiovascular system, Hypotension, Respiratory depression, Seizure, Syncope THERAPEUTIC USE: Amino Ester, Anesthetic, Local,Smoking Cessation Agent DOSE: Adult – Otitis – Topical local anesthetic, instill 4 to 5 drops of otic solution into external ear canal of affected ear(s), repeat every 1 to 2 h if necessary. Pedia – Otitis – Topical local anesthetic, (age 1 y and older) instill 4 to 5 drops of otic solution into external ear canal of affected ear(s), repeat every 1 to 2 h if necessary. CONTRA-INDICATIONS: hypersensitivity to benzocaine/ester-type local anesthetics, perforated tympanic membrane/ear discharge. ADVERSE EFFECTS: Methemoglobinemia, More common in infants and young children THERAPEUTIC USE: Anticonvulsant, Barbiturate, Long Acting, Gastrointestinal Agent, Sedative DOSE: Adult – Epilepsy, 50 mg to 100 mg ORALLY 2 or 3 times daily OR 100 mg to 320 mg slow IV injection repeated if necessary up to a MAX total dose of 600 mg/day. Pedia – Epilepsy, 15 mg to 50 mg ORALLY 2 to 3 times daily CONTRA-INDICATIONS: hypersensitivity to henobarbital products, porphyria, marked impairment of liver function, respiratory disease (dyspnea or obstruction) ADVERSE EFFECTS: Agranulocytosis, Injury of liver, Megaloblastic anemia, Osteopenia, Rickets, Scaling eczema, Stevens-Johnson syndrome, Thrombocytopenia, Thrombophlebitis THERAPEUTIC USE: Analgesic sclerosing agent, sclerosant in the treatment of hydroceles. DOSE: Up to 10mL has been injected into the tissue around internal haemorrhoids. PRECAUTIONS: Solution containing phenol should not be applied to large areas of skin or large wounds since sufficient phenol may be absorbed to give rise to toxic symptoms. THERAPEUTIC USE: Alpha-Adrenergic Blocker, Cardiovascular Agent DOSE: Adult – Pheochromocytoma, To control hypertension and sweating, initial, 10 mg ORALLY twice daily; titrate to maintenance, may increase dose every other day to 20-40 mg ORALLY 2-3 times daily. Pedia – Not FDA approved in children CONTRA-INDICATIONS: any condition compromised by hypotension, hypersensitivity to phenoxybenzamine ADVERSE EFFECTS: Seizure THERAPEUTIC USE: Alpha-Adrenergic Blocker, Antihypertensive, Diagnostic Agent, Pheochromocytoma 53 PRODUCT DESCRIPTION TRADE NAME DOSAGE FORM/STRENGTH 10mg/mL Phenylephrine HCl Mydfrin Prefrin Eye Drops 2.5% Eye Minims 10% Inj. 10mg/mL Phenytoin Epanutin Capsule 100mg Inj. 250mg/5mL Susp. 30mg/5mL Phytomenadione Konakion Inj. 2mg/0.2mL Inj. 10mg/mL Tablet 10mg Pilocarpine HCl Isopto-Carpine Eye Drops 1% Eye Drops 2% Eye Drops 4% Pinaverium Bromide Dicetel Tablet 50mg PRODUCT INFORMATION DOSE: Adult – Erectile dysfunction, 40 to 80 mg ORALLY 1 h prior to sexual activity. Hypertensive episode, Pre- or intra-operative; Treatment and Prophylaxis – Pheochromocytoma, preoperative, 5 mg IV or IM 1 to 2 h prior to surgery, may repeat as needed; intraoperative, 5 mg IV as needed. Pedia – Hypertensive episode, Pre- or intra-operative; Treatment and Prophylaxis – Pheochromocytoma; preoperative, 1 mg IV or IM 1 to 2 h prior to surgery, may repeat as needed; intraoperative, 1 mg IV as needed CONTRA-INDICATIONS: hypersensitivity to phentolamine or mannitol, myocardial infarction, coronary artery disease, angina pectoris ADVERSE EFFECTS: Cardiac dysrhythmia THERAPEUTIC USE: Adrenergic, Alkylarylamine, Anesthetic Adjunct, Decongestant, Hemorrhoidal Agent, Mydriatic-Cycloplegic, Sympathomimetic, Vasopressor DOSE: Adult – Glaucoma (open-angle glaucoma) instill 1 drop of 2.5% ophthalmic solution TOPICALLY on the upper limbus of affected eye(s) a few minutes after application of topical anesthetic; may repeat after 1 hour. Pedia – Mydriasis induction,1 drop of 2.5% ophthalmic solution to the conjunctiva; may repeat in 1 hour if necessary CONTRA-INDICATIONS: anatomical narrow-angle, hypersensitivity to phenylephrine products, infants/children of low body weight, narrow angle glaucoma, severe hypertension, tachycardia ADVERSE EFFECTS: Myocardial infarction, Pulmonary edema, Tachyarrhythmia, Ventricular arrhythmia THERAPEUTIC USE: Antiarrhythmic, Group IB, Anticonvulsant, Hydantoin (class) DOSE: Adult – Seizure, Generalized Tonic-Clonic and Complex Partial (psychomotor and temporal lobe) Seizures a)Extended-release phenytoin sodium capsules: 100 mg ORALLY 3 times a day, usual maintenance dose is 100 mg ORALLY 3-4 times a day, doses up to 200 mg ORALLY 3 times a day may be used if necessary; patients established on 100 mg 3 times a day may take one 300 mg extended-release capsules once daily b)Oral suspension: 125 mg (5 mL) 3 times daily; adjust dose every 7-10 days as necessary (MAX dose 625 mg/day) c)Oral tablets (Infatabs®): 100 mg (2 tablets) 3 times daily, usual maintenance dose 300-400 mg/day (MAX dose 600 mg/day); Status epilepticus – 10-15 mg/kg IV loading dose (not to exceed 50 mg/min), followed by maintenance doses of 100 mg ORALLY OR IV every 6-8 hr. Pedia – Seizure, Generalized Tonic-Clonic and Complex Partial (psychomotor and temporal lobe) Seizures, Extended-release phenytoin sodium capsules, oral suspension, oral tablets (Infatabs®): 5 mg/kg/day divided equally into 2 or 3 doses to a subsequent maximum of 300 mg/day; usual maintenance dose is 4 to 8 mg/kg/day; children over the age of 6 years may require minimum adult dose (300 mg/day); Status epilepticus – 15 to 20 mg/kg slow IV loading dose (not to exceed 1 to 3 mg/kg/min CONTRA-INDICATIONS: hypersensitivity phenytoin, fosphenytoin, or hydantoins, sinus bradycardia, SA block, second and third degree AV block and Adams-Stokes syndrome ADVERSE EFFECTS: Agranulocytosis, Bullous dermatosis, Granulocytopenic disorder, Leukopenia, Liver damage, Lupus erythematosus, Nephrotoxicity, Pancytopenia, Purpuric rash, Scaling eczema, Stevens-Johnson syndrome, Thrombocytopenia, Toxic epidermal necrolysis, Toxic hepatitis THERAPEUTIC USE: Nutritive Agent, Vitamin K DOSE: Adult – Drug action reversal, Anticoagulant a)2.5 to 25 mg ORALLY (rarely up to 50 mg); if prothrombin time is not satisfactory within 12 to 48 h, repeat dose b)2.5 to 25 mg IV or SUBQ (rarely up to 50 mg); if prothrombin time is not satisfactory within 6 to 8 h, repeat dose. Pedia – Hemorrhage of newborn; Prophylaxis a)(term infants), 0.5 to 1 mg IM within 1 h of birth b)(preterm infants) body weight at least 1 kg at birth, 0.5 to 1 mg IM at birth c)(preterm infants) body weight less than 1 kg at birth, 0.3 mg/kg IM CONTRA-INDICATIONS: hypersensitivity to phytonadione products ADVERSE EFFECTS: Anaphylaxis, IV and IM use THERAPEUTIC USE: Cholinergic, Dental Agent, Direct Acting Miotic DOSE: Adult – Open-angle glaucoma a)(solution) 2 drops TOPICALLY in the affected eye(s) up to 3 or 4 times per day b)(gel) 0.5 inch ribbon of 4% gel TOPICALLY in conjunctival sac once a day at bedtime c)(Ocusert Pilo®) 20 to 40 mcg/hr delivered via ocular system TOPICALLY to the conjunctiva once every 7 days. Pedia – safety and effectiveness in pediatric patients has not been established CONTRA-INDICATIONS: acute iritis or glaucoma after cataract extraction, hypersensitivity to pilocarpine products, narrow-angle (angleclosure) glaucoma, uncontrolled asthma (oral formulation) ADVERSE EFFECTS: Retinal detachment, Ophthalmic formulation THERAPEUTIC USE: Antimuscarinic, Calcium Channel Blocker DOSE: Adult – 50-milligram dose given three times daily (with an increase up to 100 milligrams three times daily if deemed necessary) is recommended by the manufacturer for irritable bowel syndrome and functional disorders of the biliary tract CONTRA-INDICATIONS: Hypersensitivity to pinaverium bromide or bromides 54 PRODUCT DESCRIPTION TRADE NAME DOSAGE FORM/STRENGTH Piperacillin/Tazobactam Tazocin Injection 4.5grams Piribedil Trivastal Tablet 50mg Plasma Proteins PPF Injection 5% Plegisol (Hospira's Cardioplegic Solution) Plegisol Solution 1 Liter Pneumococcal Polysaccharide Vaccine Pneumovax Injection 0.5mL/Dose Poliomyelitis Vaccine Oral Vaccine PRODUCT INFORMATION THERAPEUTIC USE: Antibiotic DOSE: Adult – Appendicitis (Moderate to Severe), Complicated by rupture or abscess, 3.375 g IV every 6 hours for 7 to 10 days. Pedia – Safety and efficacy in pediatric patients have not been established. Appendicitis (Moderate to Severe), Complicated by rupture or abscess a)2 to 9 months of age; 80 mg piperacillin/ 10 mg tazobactam per kg IV every 8 hours for 7 to 10 days b)9 months of age and older, up to 40 kg body weight; 100 mg piperacillin/ 12.5 mg tazobactam per kg IV every 8 hours for 7 to 10 days c)9 months of age and older, greater than 40 kg body weight; 3.375 g piperacillin/tazobactam IV every 6 hours for 7 to 10 days CONTRA-INDICATIONS: hypersensitivity to penicillins, cephalosporins or beta-lactamase inhibitors ADVERSE EFFECTS: Anaphylaxis THERAPEUTIC USE: Dopamine Agonist DOSE: Adult – Parkinson's disease, the optimal dose of piribedil in parkinsons disease is unclear. Oral doses have generally ranged from 120 to 240 mg daily. The daily dose has been given in 2 to 4 divided daily doses, although 6 to 9 doses/day have been employed by some investigators; initiated in low doses, usually 40 to 60 mg daily, with dose increments of 20 mg every 2 to 7 days. The drug is frequently administered with or after meals or with milk to improve gastrointestinal tolerance CONTRA-INDICATIONS: Hypersensitivity to piribedil THERAPEUTIC USE: Volume Expander DOSE: Adult – Hypovolemia, 12.5-25 g (250-500 mL) IV, repeat as needed. Pedia – Safety and efficacy in children not established CONTRA-INDICATIONS: cardiopulmonary bypass, congestive heart failure, hypersensitivity to albumin, hypoproteinemia associated with chronic nephrosis, chronic cirrhosis, malabsorption, protein-losing enteropathies, pancreatic insufficiency, and undernutrition, increased or normal intravascular volume, renal insufficiency, severe anemia ADVERSE EFFECTS: Hypotension, Immune hypersensitivity reaction, Shaking, chills, urticaria THERAPEUTIC USE: When suitably buffered in combination with ischemia and hypothermia is used to induce cardiac arrest during open heart surgery. DOSE: Suggested guide and is subject to variation according to the preference and experience of the surgeon. Add 10mL (840mg) of 8.4% Sodium Bicarbonate to 1liter of plegisol in order to adjust the pH. Use 10mL of Hospira list 4900, 8.4% Sodium Bicarbonate Inj. USP, to achieve the approximate pH of 7.8 when measured at Room Temp. Use of any other Sodium Bicarbonate Inj. May not achieve this pH due to the varying pH's of Sodium Bicarbonate Inj. After this addition, the solution must be used within 24hr and should be cooled to 4ºC prior to use. CONTRA-INDICATIONS: Must no be administered without the addition of 8.4% Sodium Bicarbonate Injection, USP, Hospira list 4900. ADVERSE EFFECTS: Myocardial infarction, electrocardiographic abnormalities, arrhythmias, including ventricular fibrillation. THERAPEUTIC USE: Vaccine DOSE: Adult - Pneumococcal vaccination, 23-Valent vaccine; Prophylaxis, 0.5 mL IM or SC as single dose. Pedia - Safety and effectiveness in children below the age of 2 years have not been established. Pneumococcal vaccination, 23-Valent vaccine; Prophylaxis - (2 years and older) 0.5 mL IM or SC as single dose CONTRA-INDICATIONS: hypersensitivity to pneumococcal vaccine components ADVERSE EFFECTS: Anaphylactoid reaction, Angioedema, Hemolytic anemia, Thrombocytopenia, Transfusion reaction due to serum protein reaction THERAPEUTIC USE: Vaccine DOSE: Adult - Poliomyelitis, acute; Prophylaxis a)unvaccinated adult, 0.5 mL IM or SUBQ x 3 doses given at 0, 1 to 2 mo, and 6 to 12 mo b)unvaccinated adult, less than 3 mo, but more than 2 mo available before protection is needed; 0.5 mL IM or SUBQ x 3 doses given at least 1 month apart c)unvaccinated adult, 1 to 2 mo available before protection is needed; 0.5 mL IM or SUBQ x 2 doses given at least 1 mo apart d)unvaccinated adult, less than 1 mo available before protection is needed; 0.5 mL IM or SUBQ as single dose e)incompletely vaccinated adult, 0.5 mL IM or SUBQ; additional doses needed to complete a primary series should be given if time permits f)completely vaccinated adults at increased risk of exposure, 0.5 mL IM or SUBQ as single dose. Pedia - Safety and effectiveness in infants below 6 weeks of age have not been established. Poliomyelitis, acute; Prophylaxis a)unvaccinated child, 0.5 mL IM or SUBQ x 4 doses at 2, 4, and 6 to 18 mo of age and booster at 4 to 6 years of age; do not give more frequently than 4 wks apart; first dose may be given as early as 6 wks of age b)incompletely vaccinated child, children and adolescents with a previously incomplete series should receive additional 0.5 mL IM or SUBQ doses to complete the series CONTRA-INDICATIONS: acute febrile illness; defer vaccination, anaphylaxis or anaphylactic shock occurring within 24 hrs of administration of one dose of vaccine; no further doses should be given, hypersensitivity to streptomycin, neomycin, polymixin B, hypersensitivity to polio vaccine products, hypersensitivity to 2-phenoxyethanol and formaldehyde ADVERSE EFFECTS: Anaphylaxis, Guillain-Barre syndrome, Association with vaccine unlikely, Paralytic poliomyelitis, vaccine-associated, acute 55 PRODUCT DESCRIPTION TRADE NAME DOSAGE FORM/STRENGTH Resonium A Polystyrene Na Sulphonate Polyvinyl Alcohol Sodium Resonium Liquifilm Tears Powder Eye Drops Potassium Chloride Inj. 20mEq/10mL Inj. 40mEq/20mL Powder Syrup 1mEq/mL Potassium Citrate Powder Potassium Citrate/ Sodium Citrate/Citric Acid Polycitra Solution Potassium Iodide Crystals Potassium Permanganate Crystals PRODUCT INFORMATION THERAPEUTIC USE: Exchange Resin, Hyperkalemia DOSE: Adult – Hyperkalemia, 15 g orally 1 to 4 times daily as a slurry in water or syrup; 30 to 50 g Rectally every 6hr as a warm emulsion in 100 mL aqueous vehicle (sorbitol), retain 30-60 min and follow with a cleansing enema. Pedia – Hyperkalemia, 1 g/kg/dose ORALLY every 6 hr; do not use ORALLY in neonates;1 g/kg/dose RECTALLY every 2-6 hr CONTRA-INDICATIONS: hypersensitivity to sodium polystyrene sulfonate, hypokalemia, neonates with reduced GI motility (postoperatively or drug-induced), obstructive bowel disease, oral administration in neonates ADVERSE EFFECTS: Bronchopneumonia, Electrolytes abnormal, Fecal impaction, Rectal administration, Gastrointestinal necrosis, Colonic, Hypocalcemia, Hypokalemia THERAPEUTIC USE: Ocular Lubricant, it soothes and lubricates the dry eye and is useful as an eye drop throughout the day to provide greater confort and longer wearing of hard contacts lenses. DOSE: 1 drop in the eye as needed or as directed. If irritation persists or increases discontinue use. NOTE: Not for use with soft contact lenses. Store between 15 - 30ºC. Keep container tightly closed. To avoid contamination, do not touch dropper tip to any surface. THERAPEUTIC USE: Nutriceutical, Parenteral Electrolyte, Potassium, Potassium Supplement DOSE: Adult – Hypokalemia a)serum K less than 2 mEq/L, 20 to 40 mEq/h IV, with continuous cardiac monitoring; MAX 400 mEq/day b)serum K greater than 2.5 mEq/L, 10 to 15 mEq/h IV; MAX 200 mEq/day c)serum K 3 to 3.5 mEq/L, ORAL, 40 to 100 mEq daily divided into 2 to 3 doses (no more than 20 mEq/dose); Hypokalemia; Prophylaxis, usual dose, 20 milliequivalents/day ORALLY. Pedia – Hypokalemia a)intermittent IV, 0.5 to 1 mEq/kg/dose; infuse at 0.3 to 0.5 mEq/kg/h, MAX 1 mEq/kg/h and 30 mEq per dose; MAX 3 mEq/kg/day or 40 mEq/m(2)/day b)ORAL, 2 to 5 mEq/kg/day in divided doses CONTRA-INDICATIONS: anticholinergic agents; gastrointestinal tract passage restrictions or delay may inhibit extended-release tablet passage, cardiac patients with esophageal compression; extended-release tablets may cause esophageal ulceration due to enlarged left atrium, concomitant pharmacologic agents in sufficient doses exerting anticholinergic effects; gastrointestinal tract passage restrictions or delay may inhibit extended-release tablet passage, structural or pathological conditions causing gastrointestinal tract passage restrictions or delay; may inhibit extended-release tablet passage, hyperkalemia; risk of cardiac arrest ADVERSE EFFECTS: Abdominal pain, Abnormal ECG, Cardiac arrest, Gastrointestinal ulcer, Hyperkalemia THERAPEUTIC USE: Parenteral Solution, Potassium Supplement, Urinary Alkalinizer, Urinary Stone Agent DOSE: Adult – Nephrolithiasis, mild to moderate hypocitraturia (urinary citrate greater than 150 mg/day): 10 mEq potassium citrate ORALLY three times daily with meals; severe hypocitraturia (urinary citrate less than 150 mg/day): 20 mEq potassium citrate ORALLY three times daily OR 15 mEq ORALLY four times daily; with meals or within 30 min after meals or bedtime snack CONTRA-INDICATIONS: Severe renal impairment with oliguria, azotemia, or anuria, Addison's disease, Adynamic episodica hereditaria, Acute dehydration, Heat cramps, Severe myocardial damage, Potassium citrate in patients with hyperkalemia, Sodium citrate for patients on sodium restricted diet, Potassium citrate in patients with peptic ulcer disease, Potassium citrate in patients with impaired potassium excretion, Potassium citrate in patients taking potassium-sparing diuretics, Potassium citrate in patients using salt substitutes THERAPEUTIC USE: Potassium Supplement, Urinary Alkalinizer, Urinary Stone Agent DOSE: Adult – Nephrolithiasis, mild to moderate hypocitraturia (urinary citrate greater than 150 mg/day): 10 mEq potassium citrate ORALLY three times daily with meals; severe hypocitraturia (urinary citrate less than 150 mg/day): 20 mEq potassium citrate ORALLY three times daily OR 15 mEq ORALLY four times daily; with meals or within 30 min after meals or bedtime snack CONTRA-INDICATIONS: Severe renal impairment with oliguria, azotemia, or anuria, Addison's disease, Adynamic episodica hereditaria, Acute dehydration, Heat cramps, Severe myocardial damage, Potassium citrate in patients with hyperkalemia, Sodium citrate for patients on sodium restricted diet, Potassium citrate in patients with peptic ulcer disease, Potassium citrate in patients with impaired potassium excretion, Potassium citrate in patients taking potassium-sparing diuretics, Potassium citrate in patients using salt substitutes THERAPEUTIC USE: Antibacterial Cleansing Agent, Antithyroid Agent, Expectorant, Iodine Supplement, Parenteral Mineral-Trace Mineral, Radiation Emergency, Thyroid Blocking Agent DOSE: Adult - Thyroid gland; Prophylaxis,130 mg ORALLY daily; Lymphocutaneous sporotrichosis, initial, 1 mL saturated solution ORALLY 3 times/day; increase by 0.5 mL/day to 9-12 mL/day; continue for 2-4 wk after all lesions heal. Pedia - Thyroid gland; Prophylaxis a)over 12 y and weigh 150 lbs or greater, 130 mg ORALLY daily b)over 12 y and weigh 150 lbs or less, 65 mg orally daily c)3 to 12 y, 65 mg orally daily d)1 mo to 3 y, 32.5 mg ORALLY daily e)birth to 1 mo, 16.25 mg ORALLY daily CONTRA-INDICATIONS: hypersensitivity to iodide products, renal disorders, iodine-induced goiter ADVERSE EFFECTS: Goiter, Prolonged or excessive use, Hypothyroidism, Prolonged or excessive use, Immune hypersensitivity reaction, Thyroid adenoma, Prolonged or excessive use THERAPEUTIC USE: Solutions are used as cleansing applications to wounds, ulcers, or abscesses and as wet dressing and in baths in eczematous conditions and acute dermatoses especially where there is secondary infection. Solutions have also been used in bromhidrosis, 56 PRODUCT DESCRIPTION TRADE NAME DOSAGE FORM/STRENGTH Solution 1:8000 Solution 1:10,000 Inj. 15mM/5mL Inj. 45mM/15mL Potassium Phosphate Praziquantel Biltricide Tablet 600mg Prazosin HCl Minipress Tablet 1mg Tablet 2mg Tablet 5mg Deltasone Prednisolone Gupisone Pred-Forte Primaquine Phosphate Eye Drops 1% Syrup 1mg/mL Tablet 5mg Tablet 20mg Tablet 7.5mg Procainamide HCl Pronestyl Injection 100mg/mL Procaine Benzylpenicillin Depocillin Injection 2.4mu Procarbazine Natulan Capsule 50mg PRODUCT INFORMATION in myotic infectiosn such as athlete's foot, and in poison ivy dermatitis. DOSE: Topical, 1% solution 2 to 3 times a day or in a wet dressing. ADVERSE EFFECTS: Crystals and concentrated solutions of Potassium Permanganate are caustic and even fairly dilute solutions are irritant to tissues and stain skin brown, nausea, vomiting of a brownish colored material, corrosion, edema and brown coloration of the buccal mucosa, gastrointestinal haemorrhage, liver & kidney damage and cardiovascular depression. THERAPEUTIC USE: Treatment and prevention of hypophosphatemia or hypokalemia. WARNING/PRECAUTIONS: Caution with renal insufficiency, cardiac disease, metabolic alkalosis, admixture of phosphate and calcium IV fluid can result in calcium phosphate precipitation THERAPEUTIC USE: Anthelmintic DOSE: Adult & Pedia – Schistosomiasis, 20 mg/kg ORALLY 3 times over 1 day CONTRA-INDICATIONS: Hypersensitivity to praziquantel, Ocular cysticercosis ADVERSE EFFECTS: Cardiac dysrhythmia, Heart block, Seizure THERAPEUTIC USE: Alpha-1 Adrenergic Blocker, Antihypertensive, Benign Prostatic Hypertrophy Agent, Cardiovascular Agent, ToxicologyAntidote Agent DOSE: Adult – Hypertension, initial, not to exceed 1 mg ORALLY at bedtime; maintenance, 3-20 mg ORALLY daily (divided 2-3 times daily). Pedia - Not FDA approved in children. Hypertension a)initial, 5 mcg/kg/dose ORALLY every 6 h b)titration, may increase to 25 mcg/kg/dose ORALLY c)maintenance, 25-150 mcg/kg/24 hr ORALLY (divided every 6-8 h); MAX dose, 15 mg/day or 0.4 mg/kg/day ORALLY CONTRA-INDICATIONS: hypersensitivity to prazosin or other quinazolines ADVERSE EFFECTS: Hepatotoxicity, Pancreatitis, Systemic lupus erythematosus THERAPEUTIC USE: Adrenal Glucocorticoid, Endocrine-Metabolic Agent, Immune Suppressant, Ophthalmologic Agent DOSE: Adult - Allergic disorder 5-60 mg/day ORALLY Pedia - Leprosy - Nerve injury, 1 mg/kg/day ORALLY for 1 month followed by 0.5 mg/kg/day for 3 months with further reductions of 5 mg over a month thereafter for 6 to 8 months CONTRA-INDICATIONS: Hypersensitivity, Ophthalmic products should not be used for the following: epithelial herpes simplex keratitis, acute viral infections of the cornea or conjunctiva (eg, vaccinia, varicella), fungal eye infections, mycobacterial infections of the eye, Systemic fungal infections THERAPEUTIC USE: Aminoquinoline, Antimalarial DOSE: Adult - Malaria, Prevention of relapse, 15 mg (base) orally once daily for 14 days or 45 mg (base)/week orally for 8 weeks; Malaria; Prophylaxis, 30 mg (base) orally once daily; begin 1 day before departure and continue for 7 days after leaving malarious area. Pedia Malaria, Prevention of relapse, 0.3mg/kg/day (base) orally once daily for 14 days. Malaria; Prophylaxis, 0.5 mg (base) orally once daily; begin 1 day before departure and continue for 7 days after leaving malarious area CONTRA-INDICATIONS: concomitant medications which cause bone marrow suppression, rheumatoid arthritis, lupus erythematosus, hypersensitivity to primaquine, glucose-6-phosphate dehydrogenase deficiency, pregnancy ADVERSE EFFECTS: Hemolytic anemia, Leukopenia, Methemoglobinemia THERAPEUTIC USE: Antiarrhythmic, Group IA DOSE: Adult - Advanced cardiac life support - Ventricular arrhythmia, non-VF/VT arrest, 20 mg/min IV until the arrhythmia is suppressed, hypotension ensues, or the QRS complex is prolonged by 50% from its original duration; MAX dose 17 mg/kg; maintenance infusion rate is 1 to 4 mg/min. Pedia - safety and efficacy not established in children CONTRA-INDICATIONS: complete heart block or second or third degree AV block, hypersensitivity to procainamide, procaine, or other estertype local anesthetics, systemic lupus erythematosus, torsades de pointes ADVERSE EFFECTS: Agranulocytosis, Aplastic anemia, Blood coagulation disorder, Cardiac dysrhythmia, Hepatotoxicity, Systemic lupus erythematosus THERAPEUTIC USE: Antibiotic, Penicillin, Natural DOSE: Adult – Anthrax, cutaneous, 600,000 to 1,000,000 units IM once a day; inhalational (post-exposure), 1,200,000 units IM every 12 h for 14 to 60 days. Pedia – Anthrax inhalational (post-exposure), 25,000 units/kg IM every 12 h for 14 to 60 days; MAX dose 1,200,000 units CONTRA-INDICATIONS: Hypersensitivity to penicillin/sulfites or procaine, Injection near artery or nerve ADVERSE EFFECTS: Gangrenous disorder, Due to inadvertent intravascular administration, Injection site nerve damage, Jarisch Herxheimer reaction, Myelitis, Due to inadvertent intravascular administration, Necrosis, Due to inadvertent intravascular administration, Psychotic disorder, acute, Transient THERAPEUTIC USE: Alkylating Agent, Antineoplastic Agent DOSE: Adult - Non-Hodgkin's lymphoma, COPP regimen: 100 mg/m(2)/day ORALLY on days 1-10 in combination with cyclophosphamide 57 PRODUCT DESCRIPTION Promethazine HCl Propofol Propranolol HCl TRADE NAME Histaloc Phenergan Protamine Sulphate Inj. 50mg/2mL Syrup 5mg/5mL Diprivan Inj. 200mg/20mL Inj. 500mg/50mL Inderal Inj. 1mg/mL Syrup 1mg/mL Tablet 10mg Tablet 40mg Propyl Paraben Propylthiouracil DOSAGE FORM/STRENGTH Powder PTU Tablet 50mg Injection 1% PRODUCT INFORMATION 600 mg/m(2) IV days 1 and 8; vincristine 1.4 mg/m(2) IV on days 1 and 8 and prednisone 40 mg/m(2)/day ORALLY on days 1-14 CONTRA-INDICATIONS: hypersensitivity to procarbazine, inadequate bone marrow reserve ADVERSE EFFECTS: Decreased liver function, Heinz bodies, Hemolysis, Myelosuppression, Neurotoxicity, Peripheral neuropathy, Secondary malignant neoplastic disease, Nonlymphoid THERAPEUTIC USE: Aliphatic, Antiemetic, Antihistamine, Antivertigo, Gastrointestinal Agent, Phenothiazine DOSE: Adult – Allergy a)25 mg ORALLY at bedtime or 12.5 mg ORALLY before meals and at bedtime b)25 mg IV or IM, may repeat within 2 hrs if needed. Pedia - Not for use in children less than 2 years of age. Allergy (2 yrs or older) 25 mg ORALLY at bedtime or 6.25-12.5 mg ORALLY three times daily CONTRA-INDICATIONS: Comatose states, Lower respiratory tract symptoms, including asthma, Patients with a history of an idiosyncratic reaction or hypersensitivity to promethazine or other phenothiazines, Pediatric patients less than 2 years of age, Subcutaneous or intraarterial injection ADVERSE EFFECTS: Agranulocytosis, Apnea, Jaundice, Leukopenia, Neuroleptic malignant syndrome, Respiratory depression, Thrombocytopenia THERAPEUTIC USE: Anesthetic, General DOSE: Adult - General anesthesia a)(healthy adults less than 55 years of age) induction, 40 mg IV every 10 seconds until induction onset (2 to 2.5 mg/kg); dose varies for age and surgery type b)(healthy adults less than 55 years of age) maintenance, 100 to 200 mcg/kg/min IV infusion (6 to 12 mg/kg/h); dose varies for age and surgery type c)(healthy adults less than 55 years of age) maintenance, 20 to 50 mg increments IV bolus as needed . Pedia - General anesthesia a)(3 to 16 years) induction, 2.5 to 3.5 mg/kg IV over 20 to 30 seconds b)(2 months to 16 years) maintenance, 125 to 300 mcg/kg/min IV CONTRA-INDICATIONS: hypersensitivity to propofol or its components, allergies to eggs, egg products, soybeans, or soy products ADVERSE EFFECTS: Acute renal failure, Anaphylaxis, Apnea, Bacterial septicemia, Bradyarrhythmia, Heart failure, Hypertension, Peds, Pancreatitis, Priapism, Propofol adverse reaction, Infusion syndrome, Respiratory acidosis, Seizure THERAPEUTIC USE: Antianginal, Antiarrhythmic, Group II, Antihypertensive, Antimigraine, Beta-Adrenergic Blocker, Nonselective, Cardiovascular Agent DOSE: Adult - Angina pectoris, chronic a)immediate-release, 80 to 320 mg ORALLY daily (divided 2 to 4 times/day) b)long-acting, 80 to 160 mg ORALLY once daily. Pedia - Cardiac dysrhythmia a)immediate-release, 2 to 6 mg/kg ORALLY per 24 hr (divided every 6 to 8 hr) b)immediate-release, maximum dose, 60 mg ORALLY per 24 hr c)0.1 mg/kg/dose IV, MAX 1 mg/dose, given by slow infusion over 5 min CONTRA-INDICATIONS: bronchial asthma or chronic obstructive pulmonary disease, cardiogenic shock, hypersensitivity to propranolol, overt cardiac failure, second and third degree AV block, severe sinus bradycardia ADVERSE EFFECTS: Asthma, Bronchospasm, Congestive heart failure, Erythema multiforme, Myocardial infarction, Stevens-Johnson syndrome, Toxic epidermal necrolysis THERAPEUTIC USE: An antifungal preservative, preservative for galenicals in concentrations ranging from 0.05 to 0.25%. When desired to give a strong antiseptic effect, 3 to 5 times the above concentration may be used. THERAPEUTIC USE: Antithyroid Agent, Thionamide DOSE:Adult - Hyperthyroidism a)initial, 300-400mg/day orally in divided doses every 8hrs; (initial doses as high as 600-900mg/day are necessary) b)maintenance, 100-150mg/day orally in divided doses every 8-12hrs. Pedia - safety and effectiveness not established in children under 6 years of age. Hyperthyroidism a)(6-10yrs) initial, 50-150 mg/day or 5-7 mg/kg/day ORALLY in divided doses every 6-8hrs b)(over 10yrs) initial, 150-300 mg/day or 5-7 mg/kg/day ORALLY in divided doses every 68hrs c)maintenance, 50 mg ORALLY twice daily or 1/3-2/3 of the initial dose CONTRA-INDICATIONS: hypersensitivity to propylthiouracil ADVERSE EFFECTS: Hepatic necrosis, Nephritis THERAPEUTIC USE: Heparin Antagonist DOSE: Adult - Toxicity of drug, Heparin a)1 mg IV for every 100 units of heparin remaining in patient; if 30 minutes have elapsed since the injection of heparin one-half the dose may be sufficient; maximum 50 mg given over 10 minutes b) the dose can be given as a loading dose of 25 to 50 mg by slow IV injection, with the rest of the calculated dose over 8 to 16 hours by intravenous infusion c)25 to 50 mg IV should be given immediately after the discontinuation of a continuous infusion of heparin. Pedia - safety and effectiveness in children have not been established CONTRA-INDICATIONS: hypersensitivity to protamine products ADVERSE EFFECTS: Anaphylactoid reaction, Circulatory collapse, capillary leak, noncardiogenic pulmonary edema, Anaphylaxis, Bradyarrhythmia, Hypotension 58 PRODUCT DESCRIPTION TRADE NAME Proteolytic Enzymes/ SpasmoMetixene/Dimethylpolysiloxane canulase Pyrazinamide PTB Tebrazid DOSAGE FORM/STRENGTH Tablet Syrup 100mg/mL Tablet 500mg Pyridostigmine Bromide Mestinon Inj. 1mg/mL Tablet 10mg Tablet 60mg Pyridoxine HCl Benadon Tablet 40mg Pyrimethamine /Sulphadoxine Fansidar Tablet Quinine Di-Hydrochloride/ Quinine Sulphate Inj. 300mg/mL Tablet 300mg Rabies Virus Vaccine Injection 0.5mL/Dose PRODUCT INFORMATION THERAPEUTIC CATEGORY: Use for irritable colon, digestive dysfunctions following hepatobiliary diseases, enzyme deficiencies, functional gastrointestinal disorders. DOSE: Adult & children over 12years old – swallow whole 1bitab or, in cases of acute discomfort, 2bitabs before each meal. CONTRA-INDICATIONS: Hypersensitivity to any of the components, narrow-angle glaucoma, intestinal atony. ADVERSE EFFECTS: Dry mouth, disturbed visual accommodation, tachycardia; rarely nausea, abdominal pain. THERAPEUTIC USE: Antitubercular DOSE: Adult – Tuberculosis a)(40 to 55 kg) 1,000 mg ORALLY once daily OR 1,500 mg ORALLY 3 times a week OR 2,000 mg ORALLY 2 times a week in combination with other antitubercular agents b)(56 to 75 kg) 1,500 mg ORALLY once daily OR 2,500 mg ORALLY 3 times a week OR 3,000 mg ORALLY 2 times a week in combination with other antitubercular agents c)(76 to 90 kg) 2,000 mg ORALLY once daily OR 3,000 mg ORALLY 3 times a week OR 4,000 mg ORALLY 2 times a week in combination with other antitubercular agents. Pedia Tuberculosis, 15 to 30 mg/kg ORALLY once daily (MAX 2,000 mg/day) OR 50 mg/kg ORALLY 2 times a week (MAX 4,000 mg/day) in combination with other antitubercular agents CONTRA-INDICATIONS: acute gout, hypersensitivity to pyrazinamide products, severe hepatic dysfunction ADVERSE EFFECTS: Anemia, Hepatotoxicity THERAPEUTIC USE: Central Nervous System Agent, Cholinesterase Inhibitor, Immunological Agent, Nerve Gas Antidote, Nondepolarizing Muscle Relaxant Antagonist DOSE: Adult - Myasthenia gravis a)average, 600 mg ORAL tablets or syrup daily, spaced to provide maximum relief when maximum strength is needed b)180-540 mg ORAL slow-release tablets once or twice daily, spaced at least every 6hr. Pedia - safety and effectiveness not established in children CONTRA-INDICATIONS: hypersensitivity to pyridostigmine products or bromides, mechanical intestinal obstruction, urinary obstruction ADVERSE EFFECTS: Bradyarrhythmia, Cholinergic crisis THERAPEUTIC USE: Nutritive Agent, Toxicology-Antidote Agent, Vitamin B Combination DOSE: Adult - Vitamin B6 deficiency; Treatment and Prophylaxis a)(recommended daily allowance) men and women, to 50 yr of age, 1.3 mg/day; men over 50 yr, 1.7 mg/day; women over 50, 1.5 mg/day; pregnancy 1.9 mg/day; lactation 2 mg/day b)(treatment) 10 to 20 mg/day IM or IV for 3 wk; then 2 to 5 mg/day ORALLY for several wk. Pedia - Vitamin B6 deficiency; Treatment and Prophylaxis a)(recommended daily allowance) age (0-6 months) 0.1 mg/day; (7-12 months) 0.3 mg/day; (1-3 yr) 0.5 mg/day; (4-8 yr) 0.6 mg/day; (9-13 yr) 1 mg/day; (males 14-18 yr) 1.3 mg/day; (females 14-18 yr) 1.2 mg/day b)(treatment) 5 to 25 mg/day ORALLY for 3 wk; then 1.5 to 5 mg/day ORALLY in a multivitamin preparation CONTRA-INDICATIONS: hypersensitivity to pyridoxine products THERAPEUTIC USE: Antimalarial Sulfonamide Combination DOSE: Adult - Malaria; Prophylaxis, 1 tablet ORALLY once weekly OR 2 tablets ORALLY once every 2 weeks; each tablet contains sulfadoxine 500 mg/pyrimethamine 25 mg; start 1 to 2 days before arrival in endemic area and continue during stay and for 4 to 6 wk after return. Pedia - Malaria; Prophylaxis, administer dose orally once weekly based on weight: (> 45 kg) 1 and 1/2 tablet; (31 to 45 kg) 1 tablet; (21 to 30 kg) 3/4 tablet; (11 to 20 kg) 1/2 tablet; (5 to 10 kg) 1/4 tablet; each tablet contains sulfadoxine 500 mg/pyrimethamine 25 mg; start 1 to 2 days before arrival in endemic area and continue during stay and for 4 to 6 wk after return CONTRA-INDICATIONS: blood dyscrasias, hypersensitivity to pyrimethamine or sulfonamides, infants - less than 2 months old, pregnancy or breastfeeding, severe liver or renal disease ADVERSE EFFECTS: Agranulocytosis, Aplastic anemia, Disorder of hematopoietic structure, Drug-induced eosinophilia, Hepatic necrosis, Hepatitis, Nephrotoxicity, Stevens-Johnson syndrome, Thrombocytopenia, Toxic epidermal necrolysis THERAPEUTIC USE: Antimalarial, Cinchona Alkaloid, Musculoskeletal Agent DOSE: Adult – Malaria, 2 capsules (648 mg) every 8 hours for 7 days. Pedia – Malaria over age 16 years, 2 capsules (648 mg) every 8 hours for 7 days CONTRA-INDICATIONS: glucose-6-phosphate dehydrogenase deficiency, hypersensitivity to quinine, myasthenia gravis ADVERSE EFFECTS: Disseminated intravascular coagulation, DIC, Hemolytic uremic syndrome, Hepatotoxicity, Interstitial nephritis, Ototoxicity, Thrombocytopenia THERAPEUTIC USE: Vaccine DOSE: Adult - Rabies, Post-exposure; Prophylaxis a)(not previously vaccinated) 1 mL IM x 5 doses, one each on days 0, 3, 7, 14 and 28 in conjunction with the administration of Human Rabies Immune Globulin (HRIG) on day 0; begin with the administration of HRIG 20 international units/kg IM b)(previously vaccinated) 1 mL IM x 2 doses, one each on day 0 and 3 (HRIG should not be given). Pedia - Rabies, Post-exposure; Prophylaxis a)(not previously vaccinated) 1 mL IM x 5 doses, one each on days 0, 3, 7, 14 and 28 in conjunction with the administration of Human Rabies Immune Globulin (HRIG) on day 0; begin with the administration of HRIG 20 international units/kg IM b)(previously vaccinated) 1 mL IM x 2 doses, one each on day 0 and 3 (HRIG should not be given) 59 PRODUCT DESCRIPTION TRADE NAME DOSAGE FORM/STRENGTH Ranitidine Nadine Ranid Rantag Zantac Inj. 50mg/2mL Syrup 15mg/mL Tablet 150mg Rehydration Solution Babylyte Solution 240mL Reteplase Rapilysin Injection 10units Ribavirin Rebetol Capsule 200mg Rifampicin Rifadin Capsule 150mg Capsule 300mg Syrup 20mg/mL Risperidone Risperdal Solution 1mg/mL Tablet 2mg PRODUCT INFORMATION CONTRA-INDICATIONS: hypersensitivity to processed bovine gelatin, chicken protein, neomycin, chlortetracycline and amphotericin B in trace amounts (pre-exposure), no specific contraindications (post-exposure immunization) ADVERSE EFFECTS: Anaphylaxis, Encephalitis, Guillain-Barre syndrome, Meningitis, Multiple sclerosis, Myelitis, Paralysis, Transient, Retrobulbar neuritis THERAPEUTIC USE: Antiulcer, Protectant, Gastric Acid Secretion Inhibitor, Histamine H2 Antagonist DOSE: Adult - Duodenal ulcer disease, 150 mg ORALLY twice daily or 300 mg once daily after the evening meal or at bedtime; 50 mg IV/IM every 6-8 hours or 6.25 mg/h IV continuous infusion.Pedia - Duodenal ulcer disease a)(1 month of age or older) 2 to 4 mg/kg ORALLY twice daily, MAX 300 mg/day b)(1 month of age or older) 2 to 4 mg/kg/day IV in divided doses every 6-8 hours, MAX 50 mg every 6-8 hours. CONTRA-INDICATIONS: hypersensitivity to ranitidine or any of its ingredients ADVERSE EFFECTS: Anemia, Necrotizing enterocolitis in fetus or newborn, Pancreatitis, Thrombocytopenia THERAPEUTIC USE: Indicated as a maintenance fluid for infants and young children to prevent dehydration in mild or moderate diarrhea. DOSE: Children under 2years of age – before use consult the physician; Children over 2years of age – Offer babylyte every 3 or 4hours. One to two liters should be taken each day while the diarrhea continues. If the diarrhea continues for more than 24hours or there is vomiting or fever consult your doctor. CONTRA-INDICATIONS: Patients with intractable vomiting, adynamic ileus, intestinal obstruction or bowel perforation. THERAPEUTIC USE: Blood Modifier Agent, Tissue Plasminogen Activator DOSE: Adult - Acute myocardial infarction, 10 unit IV bolus, 2 doses given 30 minutes apart. Pedia - safety and efficacy in children have not been established CONTRA-INDICATIONS: hypersensitivity to reteplase, active internal bleeding, severe uncontrolled hypertension, recent intracranial or spinal surgery or trauma, known bleeding diathesis, history of cerebrovascular accident, intracranial neoplasm, arteriovenous malformation, or aneurysm ADVERSE EFFECTS: Cardiac dysrhythmia, Reperfusion, Cholesterol embolus syndrome, Gastrointestinal hemorrhage, Immune hypersensitivity reaction, Intracranial hemorrhage THERAPEUTIC USE: Antiviral, Guanosine Nucleoside Analog, Viral RNA Polymerase Inhibitor DOSE: Adult - Hepatitis C, chronic, In patients with compensated liver disease a)in combination with interferon alfa-2b, 75 kg or less, 400 mg ORALLY every morning and 600 mg ORALLY every evening b)in combination with interferon alfa-2b, greater than 75 kg, 600 mg ORALLY twice a day c)in combination with peg-interferon alfa-2b, 400 mg ORALLY every morning and 400 mg ORALLY every evening. Pedia - Hepatitis C, chronic, In patients with compensated liver disease. a)greater than 61 kg, use adult dosing b)in combination with interferon alfa-2b, 25 to 36 kg, 200 mg ORALLY twice a day c)in combination with interferon alfa-2b, 37 to 49 kg, 200 mg ORALLY every morning and 400 mg ORALLY every evening d)in combination with interferon alfa-2b, 50 to 61 kg, 400 mg ORALLY twice a day CONTRA-INDICATIONS: cardiac disease, significant or unstable; potential worsening due to drug-induced anemia, pregnancy or pregnant partner of male patient; may cause birth defects and/or death of the exposed fetus, hemoglobinopathy (such as thalassemia major and sicklecell anemia), hepatic decompensation, in cirrhotic chronic hepatitis C patients coinfected with HIV before or during therapy, hepatitis, autoimmune; ribavirin may worsen hepatitis, hypersensitivity to ribavirin or any component of the product, renal function impairment ADVERSE EFFECTS: Bacterial infectious disease, oral, in combination with peginterferon alfa-2a, Bradyarrhythmia, Inhalation, Cardiac arrest, Complication of respiratory therapy procedure, Drug precipitation, Hemolytic anemia, Cardiac and pulmonary events have occurred, Hepatotoxicity, Hyperammonemia, Hyperbilirubinemia, Hypotension, Increased erythrocyte destruction, Liver failure, oral, in combination with peginterferon alfa 2a, Pancreatitis, Fatal and nonfatal, Respiratory complication, Suicide, oral, in combination with peginterferon alfa-2a, Thrombotic thrombocytopenic purpura THERAPEUTIC USE: Antitubercular, Rifamycin DOSE: Adult – Tuberculosis, initially, 10 mg/kg/day (in combination with isoniazid and pyrazinamide) ORALLY or IV for 2 mo; MAX 600 mg/day; then 10 mg/kg/day (in combination with isoniazid) for 4 mo or longer as needed. Pedia – Tuberculosis, initially, 10 to 20 mg/kg/day (in combination with isoniazid and pyrazinamide) ORALLY or IV for 2 mo; MAX 600 mg/day; then 10 to 20 mg/kg/day (in combination with isoniazid) for 4 mo or longer as needed. CONTRA-INDICATIONS: hypersensitivity to rifampin or other rifamycins, presence of active Neisseria meningitidis infection ADVERSE EFFECTS: Hepatotoxicity, Thrombocytopenia, High-dose therapy THERAPEUTIC USE: Antipsychotic, Benzisoxazole DOSE: Adult -Manic bipolar I disorder, a)(monotherapy or in combination with lithium or valproate) initial, 2 to 3 mg ORALLY once a day b)(monotherapy or in combination with lithium or valproate) maintenance, dosage adjustments should be made in increments of 1 mg/day at intervals of at least 24 hours; doses higher than 6 mg/day have not been evaluated in clinical trials. Pedia - safety and effectiveness in pediatric patients less than 13 years of age with schizophrenia or less than 10 years with bipolar mania have not been established, safety and 60 PRODUCT DESCRIPTION TRADE NAME DOSAGE FORM/STRENGTH Ritodrine HCl Yutopar Injection 50mg/5mL Rocuronium Bromide Esmeron Injection 50mg/5mL Rubella Vaccine Salbutamol Injection 0.5mL Butalin Ventolin Salicylic Acid Salicylic Acid/Lactic Acid /Lauromacrogol Scorpion Antivenom Inhaler 100mcg Solution 5mg/mL Powder Collomack Solution Injection PRODUCT INFORMATION effectiveness in pediatric patients less than 5 years of age with autistic disorder have not been established; Manic bipolar I disorder - (10 years and older) initial, 0.5 mg ORALLY once daily as a single dose in the morning or evening; adjust dosage at intervals not less than 24 hours and in increments of 0.5 to 1 mg/day up to a maximum recommended dose of 2.5 mg/day CONTRA-INDICATIONS: hypersensitivity to risperidone or to any product component ADVERSE EFFECTS: Death, Hypothermia, Leukopenia, Neuroleptic malignant syndrome, Pancreatitis, Priapism, Purpuric disorder, Seizure, Suicidal intent, Syncope, Tardive dyskinesia, Thrombocytopenia THERAPEUTIC USE: Beta-2 Adrenergic Agonist, Uterine Relaxant DOSE: Adult - IV route, For use in PREMATURE LABOR, the manufacturer recommends an initial dose of 0.05 mg (50 mcg)/min. The dose is gradually increased by 0.05 mg (50 mcg/min every 10 min until the desired response is attained. The effective dose is usually between 150 to 350 mcg/min. The infusion should be continued for 12 to 24 hours after uterine contractions cease. Ten mg orally may be administered 30 min before the termination of the infusion, and then every 2 hours for the next 24 hours. Thereafter 10 to 20 mg every 4 to 6 hours can be given for as long as it is desirable to prolong the pregnancy. Maximum dose - Intravenous infusions of ritodrine should not exceed 350 mg/min CONTRA-INDICATIONS: Contraindicated for use before the 20th week of pregnancy, Hypersensitivity to ritodrine, Pre-existing maternal medical conditions such as hypovolemia, cardiac arrhythmias associated with tachycardia or digitalis intoxication, uncontrolled hypertension, pheochromocytoma, and bronchial asthma already treated by beta-mimetics and/or steroids, Conditions of the mother or fetus in which continuation of pregnancy would be hazardous; including antepartum hemorrhage, eclampsia and severe pre-eclampsia, intrauterine fetal death, chorioamnionitis, maternal cardiac disease, pulmonary hypertension, maternal hyperthyroidism, and uncontrolled maternal diabetes mellitus THERAPEUTIC USE: Musculoskeletal Agent, Neuromuscular Blocker, Non-Depolarizing DOSE: Adult - General anesthesia; Adjunct - Induction of neuromuscular blockade, During surgery or mechanical ventilation, initial, 0.6 mg/kg IV, maintenance, 0.1 to 0.2 mg/kg IV push, repeat as needed OR 0.01 to 0.012 mg/kg/minute continuous IV infusion. Pedia - General anesthesia; Adjunct - Induction of neuromuscular blockade, During surgery or mechanical ventilation, 3 mo to 14 y, initial, 0.6 mg/kg/dose IV; 3 mo to 14 y, maintenance, 0.075 to 0.125 mg/kg IV push as needed OR 0.012 mg/kg/min continuous IV infusion. CONTRA-INDICATIONS: hypersensitivity to rocuronium products ADVERSE EFFECTS: Anaphylaxis, Cardiac dysrhythmia, Hypertension, Hypotension, Tachyarrhythmia THERAPEUTIC USE: Vaccine DOSE: Adult - Prophylaxis, 0.5 mL SC x 1 dose. Pedia - safety and effectiveness in infants below the age of 12 mo have not been established. Rubella; Prophylaxis, (12 mo and older) 0.5 mL SC x 1 dose CONTRA-INDICATIONS: Active febrile infection, Acute febrile illness, Active, untreated TB, Blood dyscrasias, leukemia, lymphomas, bone marrow malignancy, Family history of congenital or hereditary immunodeficiency, Anaphylactic reactions to topical or systemic neomycin, Hypersensitivity to vaccine components, including gelatin, Primary or acquired immunodeficiency including immunocompromised HIV, Immunosuppressive therapy, Pregnancy should be avoided for 28 days postvaccination based on Advisory Committee on Immunization Practices ADVERSE EFFECTS: Anaphylaxis, Encephalitis, Guillain-Barre syndrome, Association with vaccine unlikely, Optic neuritis, Polyneuritis, Polyneuropathy, Stevens-Johnson syndrome, Thrombocytopenia THERAPEUTIC USE: Beta-2 Adrenergic Agonist, Bronchodilator, Cardiovascular Agent, Sympathomimetic DOSE: Adult - Asthma; Treatment and Prophylaxis, 2 INHALATIONS every 4-6 h or 1 INHALATION every 4 h; Exercise-induced asthma; Prophylaxis, 2 INHALATIONS 15 min before exercise. Pedia - Asthma; Treatment and Prophylaxis, 4 y and older, 2 INHALATIONS every 4-6 h or 1 INHALATION every 4 h; Exercise-induced asthma; Prophylaxis, 4 y and older, 2 INHALATIONS 15 min before exercise CONTRA-INDICATIONS: hypersensitivity to albuterol or any of its components ADVERSE EFFECTS: Erythema multiforme, Stevens-Johnson syndrome THERAPEUTIC USE: Analgesic, Antiacne Keratolytic, Antipsoriatic, Antipyretic, Antirheumatic, Antiseborrheic, Keratolytic, NSAID, Salicylate, Non-Aspirin DOSE: Adult - Acne vulgaris, apply TOPICALLY in concentrations of 0.5% to 10%; Psoriasis - apply TOPICALLY in concentrations of 3% to 6%; Removal of wart- apply TOPICALLY once or twice daily in concentrations of 5% to 40% for up to 12 weeks Pedia – Psoriasis, apply TOPICALLY in concentrations up to 6%. CONTRA-INDICATIONS: age less than 2 yrs, diabetes, hypersensitivity to salicylic acid, impaired circulation THERAPEUTIC USE: Keratolytic, anti callous preparations, corns, hard skins and for warts. DOSE: Apply 1drop night and morning to affected area only for several days. ADVERSE EFFECTS: Local irritation THERAPEUTIC USE: The polyvalent antivenom is highly specific in neutralizing the venoms of the Saudi yellow scorpion and black scorpion. The antivenow has also a wide spectrum of activity and can neutralize the venoms of many of the Middle east and North African scorpions. 61 PRODUCT DESCRIPTION TRADE NAME DOSAGE FORM/STRENGTH Laxal Senna Glycosides Pursennid Tablet 12mg Sennalax Sevoflurane Sevorane Inhalation Silver Nitrate Grafco Stick 95% Silver Sulphadiazine Flamazine Cream 1% Simvastatin Simvast Zocor Tablet 10mg Tablet 20mg Snake Antivenom Serum Injection Sodium Bicarbonate Injection 4.2% Injection 8.4% Powder Syrup 1mEq/mL Tablet 325mg PRODUCT INFORMATION DOSE: Adult & Pedia – Five 1mL ampoules are to be diluted in 20-50mL half normal saline and infused IV slowly over a period of 30minutes. The dose is to be repeated if necessary, up to 20 x 1mL ampoules. One quarter normal saline is to be used for infants & children up to 6years old. Children must be given the same dose of antivenom as adults. ADVERSE EFFECTS: Anaphylactic reaction THERAPEUTIC USE: Anthraquinone laxatives are used primarily for short-term treatment of constipation and to evacuate the colon for rectal and bowel examination. DOSE: Adult – usual recommended dose is sennosides 15 to 17 milligrams (mg) once a day, ideally before bedtime, up to a maximum of 34 to 50 mg twice a day. Pedia – Ages 2 to 6 The recommended initial dosage in children between 2 and 6 years of age is 4.3 mg sennosides once a day, preferably at bedtime. The maximum recommended dosage is 8.6 mg sennosides twice a day; Ages 6 to 12 – recommended initial dosage in children between 6 and 12 years of age is 8.5 to 15 mg sennosides once a day, preferably at bedtime. The maximum recommended dosage is 17 to 25 mg sennosides twice a day. CONTRA-INDICATIONS: Stimulant laxatives are contraindicated in patients with the following conditions: acute surgical abdomen; bowel obstruction; fecal impaction; hypersensitivity to anthraquinone laxatives or to any of the ingredients; nausea, vomiting, or other symptoms of appendicitis; or undiagnosed abdominal pain. THERAPEUTIC USE: Haloalkane, Volatile Liquid DOSE: Adult – General anesthesia, 0.5% to 3% concentration with or without concomitant use of nitrous oxide. Pedia – General anesthesia, 0.5% to 3% concentration with or without concomitant use of nitrous oxide CONTRA-INDICATIONS: hypersensitivity to sevoflurane or other halogenated agents, malignant hyperthermia, known or suspected susceptibility; sevoflurane may trigger reaction ADVERSE EFFECTS: Bradyarrhythmia, Hepatic necrosis, Hypotension, Laryngeal spasm, Liver failure, Malignant hyperthermia, Respiratory depression, Seizure, Tachyarrhythmia THERAPEUTIC USE: Antibacterial, Antiseptic, Dressing Agent, Keratolytic, Trace Mineral Supplement DOSE: Adult – Chronic granulomatous disease, TOPICAL, apply 10% solution with cotton applicator to wart or lesion 2-3 times per wk for 2-3 wk as needed. TOPICAL, apply ointment to affected area and cover with apertured pad for 5 days. Pedia – Gonococcal conjunctivitis neonatorum, 2 drops of 1% solution each eye immediately after delivery CONTRA-INDICATIONS: hypersensitivity to silver nitrate products ADVERSE EFFECTS: Methemoglobinemia THERAPEUTIC USE: Antibacterial, Sulfonamide DOSE: Adult – Burn, TOPICAL, apply 1% cream in 1/16-inch layer once or twice daily until healing or skin grafting. Pedia – safety and effectiveness not established in children CONTRA-INDICATIONS: hypersensitivity to silver or sulfonamide products, pregnancy (approaching term), preterm infants or newborns under 2 months ADVERSE EFFECTS: Disorder of hematopoietic structure, Leukopenia THERAPEUTIC USE: Antihyperlipidemic, HMG-COA Reductase Inhibitor DOSE: Adult – Hypercholesterolemia, initial, 20 to 40 mg orally once every evening; dose range, 5 to 80 mg ORALLY daily. Pedia – safety and efficacy have not been studied in pediatric patients under 10 years of age. Familial hypercholesterolemia – heterozygous, adolescents 10 to 17 years of age: initial, 10 mg orally daily in the evening; maintenance, 10 to 40 mg orally daily; MAX 40 mg/day CONTRA-INDICATIONS: active liver disease or elevated liver enzymes, hypersensitivity to simvastatin products, pregnancy and lactation ADVERSE EFFECTS: Compartment syndrome of lower leg, Drug-induced myopathy, Hepatotoxicity, Rhabdomyolysis, Rupture of tendon THERAPEUTIC USE: The polyvalent antivenom is highly specific in neutralizing the toxic effects of the six Saudi snake venoms. Neutralize effectively the haemorrhagic and myonecrotic activities of the viper snake venoms and the neuromascular blocking and cardiotoxic effects of the elapid snake venoms. DOSE: Adult & Pedia – 40mL antivenom are diluted in approximately 5mL isotonic physiological fluid,/Kg body weight and infused IV slowly over a period of 30 – 60 minutes. Alternatively, IV undiluted at a rate of 4mL/min. Children must be given the same dose as Adults. ADVERSE EFFECTS: Allergic Reactions. THERAPEUTIC USE: Antacid, Sodium Bicarbonate Containing, Parenteral Electrolyte, Sodium, Sodium Supplement DOSE: Adult – Oral route, Sodium bicarbonate 1 gram equals 11.9 milliequivalents sodium and 11.9 milliequivalents bicarbonate. The usual adult dose is 325 milligrams to 2 grams orally 4 times per day. Maximum daily dose in patients less than 60 years is 16 grams and greater than 60 years is 8 grams/day; IV route, a)Hypertonic solutions of sodium bicarbonate must be diluted to isotonicity (1.5%) with sterile water, sodium chloride, dextrose 5%, or other standard electrolyte solutions before administration b)Undiluted hypertonic 8.4% sodium bicarbonate may be given by intravenous injection during cardiac arrest c)Flush intravenous lines before and after sodium bicarbonate infusion. Pedia – 62 PRODUCT DESCRIPTION TRADE NAME DOSAGE FORM/STRENGTH Tablet 500mg Sodium Chloride Inj. 2.5mEq/mL Inj. 5.13mEq/mL Powder Syrup 2.5mEq/mL Sodium Citrate Powder Sodium Cromoglycate Opticrom Eye Drops 2% Sodium Hyaluronate Healon Injection 10mg/mL Sodium Nitroprusside Sodium Phosphate Nipride Nipruss Injection 50mg/2mL Injection 3mM/mL PRODUCT INFORMATION IV route a)In pediatric patients, use of sodium bicarbonate is recommended for symptomatic patients with HYPERKALEMIA, HYPERMAGNESEMIA, TRICYCLIC ANTIDEPRESSANT OVERDOSE, or overdose from other sodium channel blocking agents and may be considered for the patient with PROLONGED CARDIAC ARREST or shock associated with documented severe METABOLIC ACIDOSIS. Pediatric dosing recommendations include an initial dose of 1 milliequivalents/kilogram (1 milliliter (mL)/kg of 8.4% solution) intravenously or intraosseously. To limit the osmotic load in neonates, a dilute solution (0.5 mEq/mL; 4.2% solution) may be used. Subsequent doses should be based on blood gas analysis or after every 10 minutes of continued arrest b)For neonates and children under 2 years, do not administer more than 8 milliequivalents/kilogram/day; use a 4.2% solution or dilute the 8.4% solution to 0.5 milliequivalent/milliliter for slow administration. Rapid injection (10 milliliter/minute) may produce hypernatremia, a decrease in cerebrospinal fluid pressure and possible intracranial hemorrhage CONTRA-INDICATIONS: Respiratory alkalosis, Hypocalcemia, Hypochloremia, Do not use Neutralizing Additive Solution 4.2% as a systemic alkalinizer THERAPEUTIC USE: Antiasthma, Dermatological Agent, Dressing Agent, Eye Wash Irrigant, Moisturizer, Nutriceutical, Osmotherapy Agent Parenteral Electrolyte, Sodium, Respiratory Agent, Sodium Supplement, Wound Care Agent DOSE: Adult – Aldosterone measurement, urine a)Oral doses used have included: 12 gm/d ORALLY for 3 d, b)25 ml/kg of normal saline IV infused over 4 hours for 3 d; Plasma aldosterone level; Intravenous doses used have included: 2-3 L of normal saline IV infused over 4-6 h CONTRA-INDICATIONS: Hypernatremia or fluid retention, Hypersensitivity, Sodium chloride solutions with preservatives in newborns, for injection or flushing of intravenous lines or mixing medications, Hypertonic saline abortifacient in pregnancies less than 15 weeks or more than 24 weeks gestation or in presence of increased intra-amniotic pressure, as with contractions or hypertonic uterus, Hypertonic saline abortifacient in patients with major systemic disorders, such as diabetes mellitus, renal, hepatic, or sickle cell disease; prior to uterine or cervical surgery; in the presence of pelvic adhesions THERAPEUTIC USE: Urinary Alkalinizer, Urinary Stone Agent and mild astringent THERAPEUTIC USE: Antiasthma, Gastrointestinal Agent, Mast Cell Stabilizer, Nasal Agent, Ophthalmologic Agent DOSE: Adult - Allergic rhinitis, NASAL INHALATION, 1 spray/nostril 3-6 times/day; Asthma; Prophylaxis, ORAL INHALATION, 20 mg 4 times/day (nebulizer) or 2 puffs (800 mcg/spray) 4 times/day (metered inhaler), then 3-4 times/day. Pedia - Allergic rhinitis, NASAL INHALATION (2 y and older), 1 spray/nostril 3-6 times/day, Asthma; Prophylaxis, ORAL INHALATION, 20 mg 4 times/day (2 years and older; nebulizer) or 2 puffs (800 mcg/spray) 4 times a day (5 years and older; metered inhaler), then 3-4 times/day CONTRA-INDICATIONS: hypersensitivity to cromolyn products ADVERSE EFFECTS: Anaphylaxis, Bronchospasm THERAPEUTIC USE: Cartilaginous Defect Repair Agent, Dermatological Agent, Ophthalmologic Agent, Protectant, Dermatological, Surgical Aid, Ocular DOSE: Adult - Cataract surgery; Adjunct a sufficient amount of sodium hyaluronate is slowly and carefully introduced (using a cannula or needle) into the anterior chamber; injection can be performed either before or after delivery of the lens. Pedia - safety and effectiveness in children have not been established. CONTRA-INDICATIONS: hypersensitivity to hyaluronan, active knee joint infections or skin disease at injection site, allergy to avian proteins, feathers, and egg products ADVERSE EFFECTS: Inflammatory disorder, Postoperative, including iritis, hypopyon, endophthalmitis, Pseudogout, Raised intraocular pressure, Postoperative, likely if hyaluronate not removed as completely as possible after surgery, Septic arthritis THERAPEUTIC USE: Antihypertensive, Peripheral Vasodilator, Coronary Vasodilator, Peripheral Vasodilator DOSE: Adult & Pedia - Congestive heart failure, initial, 0.3 mcg/kg/min IV infusion, titrate every few min to desired effect; usual dose, 3 mcg/kg/min IV; MAX dose 10 mcg/kg/min. CONTRA-INDICATIONS: compensatory hypertension (aortic coarctation or atriovenous shunting), congestive heart failure associated with reduced peripheral vascular resistance, hypersensitivity to nitroprusside, optic atrophy, surgery patients with inadequate cerebral circulation, symptomatic hypotension, tobacco amblyopia ADVERSE EFFECTS: Bowel obstruction, Cardiac dysrhythmia, Cyanide poisoning, Hemorrhage, Excessive, decreased platelet aggregation, Hypotensive episode, Excessive, Metabolic acidosis, Methemoglobinemia, Raised intracranial pressure, Toxicity of drug, Thiocyanate THERAPEUTIC USE: Indicated as a source of Phosphorous, for addition to large volume IV fluids, to prevent or correct hypophosphatemia in patients with restricted or no oral intake. It is also useful as an additive for preparing specific parenteral fluid formulas when the needs of the patient cannot be met by standard electrolyte or nutrient solutions. DOSE: The dose and rate of administration are dependent upon the individual needs of the patient. Serum sodium, phosphorus and calcium levels should be monitored as a guide to dosage. In TPN approximately 12 to 15 mM of Phosphorus (equivalent to 372 to 465mg elemental 63 PRODUCT DESCRIPTION TRADE NAME DOSAGE FORM/STRENGTH Sodium Stibogluconate Pentostam Injection 100mg/mL Sodium Tetradecyl Sulphate STD Injection 3% Depakine Syrup 200mg/3.5mL Tablet 200mg Tablet 500mg Sodium Valproate Depakine Chrono Spironolactone Aldactone Syrup 1mg/mL Tablet 25mg Tablet 100mg Sterculia Normacol Plus Granules 62% PRODUCT INFORMATION phosphorus) per liter bottle of TPN solution containing 250 grams dextrose is usually adequate to maintain normal serum phosphorus. For infants receiving TPN is 1.5 to 2 mM P/Kg/Day. CONTRA-INDICATIONS: High Phosphorus or low calcium levels may be encountered and in patients with hypernatremia. ADVERSE EFFECTS: Possible Phosphorus intoxication results in a reduction of serum calcium and the symptoms are those hypocalcemic tetany. THERAPEUTIC USE: Cutaneous leishmaniasis, visceral leishmaniasis DOSE: Adult - 10-day course (n=19) of sodium stibogluconate 20 mg/kg/day was as effective as a 20-day course in the treatment of cutaneous leishmaniasis. Pedia - Recommended intravenous or intramuscular doses of sodium stibogluconate in children are 20 mg of antimony/kg IV or IM once daily for 20 to 28 days. A longer duration may be necessary for some forms of VISCERAL LEISHMANIASIS, or the African form of the disease CONTRA-INDICATIONS: Hypersensitivity to SODIUM STIBOGLUCONATE THERAPEUTIC USE: Sclerosing Agent DOSE: Adult - Venous varices, 0.5 to 2 mL (preferably 1 mL) IV into the vein intended for sclerosis; the 1% solution is used most often, with the 3% solution being reserved for larger varicosities; MAX single treatment, do not exceed 10 mL CONTRA-INDICATIONS: Acute cellulitis, Acute infections, Acute superficial thrombophlebitis, Allergic conditions, Bedridden patients, Huge superficial veins with wide open communications to deeper veins, Phlebitis migrans, Previous hypersensitivity reactions to the drug, Significant valvular or deep vein incompetence, Uncontrolled systemic diseases, Varicosities caused by abdominal and pelvic tumors unless the tumor has been removed ADVERSE EFFECTS: Anaphylaxis, death reported, Deep venous thrombosis, Necrosis, following extravasation, Pulmonary embolism, Skin necrosis, following extravasation THERAPEUTIC USE: Anticonvulsant, Antimigraine, Valproic Acid (class) DOSE: Adult - Complex partial epileptic seizure a)monotherapy, 10 to 15 mg/kg/day IV, may increase dosage 5 to 10 mg/kg/week to achieve optimal clinical response (MAX 60 mg/kg/day or less with a therapeutic range of 50 to 100 mcg/mL) b)conversion to monotherapy, 10 to 15 mg/kg/day IV, may increase dosage 5 to 10 mg/kg/week to achieve optimal clinical response (MAX 60 mg/kg/day or less with a therapeutic range of 50 to 100 mcg/mL); reduce concomitant antiepilepsy drug dosage by approximately 25% every 2 weeks (reduction may be started at initiation of therapy or delayed by 1 to 2 weeks if there is a concern that seizures are likely to occur with a reduction) c)adjunct, may be added to the patient's regimen at an initial dosage of 10 to 15 mg/kg/day IV, may increase dosage 5 to 10 mg/kg/week to achieve optimal clinical response (MAX 60 mg/kg/day or less with a therapeutic range of 50 to 100 mcg/mL). Pedia - safety and effectiveness in pediatric patients under age 10 have not been established; for children 10 years of age and older, dose as an adult. Complex partial epileptic seizure a)(10 yrs and older) monotherapy, 10 to 15 mg/kg/day IV, may increase dosage 5 to 10 mg/kg/week to achieve optimal clinical response (MAX 60 mg/kg/day or less with a therapeutic range of 50 to 100 mcg/mL) b)(10 yrs and older) conversion to monotherapy, 10 to 15 mg/kg/day IV, may increase dosage 5 to 10 mg/kg/week to achieve optimal clinical response (MAX 60 mg/kg/day or less with a therapeutic range of 50 to 100 mcg/mL); reduce concomitant antiepilepsy drug dosage by approximately 25% every 2 weeks (reduction may be started at initiation of therapy or delayed by 1 to 2 weeks if there is a concern that seizures are likely to occur with a reduction) c)(10 yrs and older) adjunct, may be added to the patient's regimen at an initial dosage of 10 to 15 mg/kg/day IV, may increase dosage 5 to 10 mg/kg/week to achieve optimal clinical response (MAX 60 mg/kg/day or less with a therapeutic range of 50 to 100 mcg/mL) CONTRA-INDICATIONS: hepatic disease or significant hepatic dysfunction, hypersensitivity to valproate sodium, valproic acid, or divalproex sodium, known urea cycle disorders; hyperammonemic encephalopathy, including fatalities, has occurred ADVERSE EFFECTS: Liver failure, Children under the age of two years are at increased risk, Pancreatitis, Life-threatening, Thrombocytopenia, Dose-related THERAPEUTIC USE: Aldosterone Receptor Antagonist, Antiandrogen, Cardiovascular Agent, Diuretic, Potassium Sparing DOSE: Adult - Edema - Nephrotic syndrome, initial, 100 mg/day ORALLY (range 25 to 200 mg/day) in single or divided doses; after 5 days, adjust dosage (MAX 400 mg/day); then if response is inadequate after 5 days, consider adding another diuretic; in some patients, alternateday dosing has been effective. Pedia – NEONATES a)The suggested dosage regimen of spironolactone is 0.5 to 1 mg/kg orally every 8 hours; CHILDREN Usual oral dose of spironolactone in children is 1 to 3 mg/kg/day (1.5 mg/lb) in a single or 2 to 4 divided doses up to a maximum dose of 200 milligrams/24 hours. The dose should be reduced to 1 to 2 mg/kg for maintenance or when used in combination with other diuretics CONTRA-INDICATIONS: anuria, hyperkalemia, hypersensitivity to spironolactone products, renal insufficiency, acute ADVERSE EFFECTS: Agranulocytosis, Breast cancer, Cause and effect not established, Gastric hemorrhage, Gastritis, Hyperkalemia (Severe), Metabolic acidosis, Skin ulcer, Systemic lupus erythematosus THERAPEUTIC USE: Treatment of constipation, particularly simple or idiopathic constipation and constipation arising in pregnancy. Management of colostomies and ileostomies. 64 PRODUCT DESCRIPTION Streptokinase TRADE NAME Streptase Streptomycin DOSAGE FORM/STRENGTH Injection 750,000units Injection 1gram Gastrofatt Sucralfate Ulcar Tablet 500mg Ulsaheal Sucrose Sufentanil Powder Sufenta Forte Injection 0.25mg/5mL Blephamide Sulphacetamide Isopto-Cetamide Eye Drops 10% Eye Drops 15% Riacetamide PRODUCT INFORMATION DOSE: Adult – 1 or 2 sachets once or twice daily after meals. Pedia - (6 to 12 years) one half of the adult dose; Under 6 years – only at the discretion of the doctor. The granules should be placed dry on the tongue and, without chewing or crushing, swallowed immediately with plenty of liquid (water or a cool drink). CONTRA-INDICATIONS: Intestinal obstruction, faecal impaction and total atony of the colon. ADVERSE EFFECTS: Occasionaly mild abdominal distention, oesophageal obstruction is possible if the product is taken in overdosage or if it is not adequately washed down with fluid. THERAPEUTIC USE: Thrombolytic, Tissue Plasminogen Activator DOSE: Adult - Thromboembolic disorder, Arterial, loading dose, 250,000 international units IV over 30 min, then 100,000 international units/h for 24 hr. Pedia - Thromboembolic disorder, Arterial, loading dose, 1000 international units/kg IV over 5-30 min; continuous infusion, 1000 international units/kg/h for up to 24 h CONTRA-INDICATIONS: hypersensitivity to streptokinase products, severe uncontrolled hypertension, active internal bleeding, recent intracranial or intraspinal surgery or trauma (within 2 months), intracranial neoplasm, arteriovenous malformation, or aneurysm, known bleeding diathesis, history of cerebrovascular accident (within 2 months) ADVERSE EFFECTS: Anaphylactoid reaction, Anaphylaxis, Bleeding, Major, Bradyarrhythmia, Cardiac dysrhythmia, Reperfusion, Cholesterol embolus syndrome, Non-cardiogenic pulmonary edema, Polyneuropathy, Tachyarrhythmia THERAPEUTIC USE: Aminoglycoside, Antibiotic, Antitubercular DOSE: Adult – Tuberculosis, 15 mg/kg/day IM once a day (maximum 1 g/day), or 25-30 mg/kg/dose 2-3 times weekly (maximum 1.5 g/dose) in combination with other antitubercular antibiotics for the first 2 months of therapy; maximum total dose 120 g/course. Pedia –Tuberculosis, 20-40 mg/kg IM once a day (maximum 1 g/day), or 25-30 mg/kg IM 2-3 times weekly (maximum 1.5 g/day) in combination with other antitubercular antibiotics for the first 2 months of therapy; maximum total dose 120 g/course. CONTRA-INDICATIONS: hypersensitivity to streptomycin/aminoglycosides, hypersensitivity to sulfites ADVERSE EFFECTS: Arachnoiditis, Disorder of inner ear, Disorder of optic nerve, Encephalopathy, Nephrotoxicity, Neuromuscular blockade finding Concomitant anesthesia, muscle relaxants, Peripheral neuritis, Respiratory tract paralysis Concomitant anesthesia, muscle relaxants THERAPEUTIC USE: Antiulcer, Protectant DOSE: Adult - Duodenal ulcer disease, Active, treatment, 1 gram ORALLY 4 times daily or 2 grams twice daily for 4 to 8 weeks. Pedia safety and efficacy in pediatric patients not established. Duodenal ulcer disease, Active, treatment, 40 to 80 mg/kg/day ORALLY divided every 6 hours OR 0.5 to 1 gram 4 times daily CONTRA-INDICATIONS: hypersensitivity to sucralfate products ADVERSE EFFECTS: Aluminum toxicity, Renal impaired patients, Bezoar THERAPEUTIC USE: Principally as a pharmaceutical necessity for making syrups and lozenges. It gives viscosity and consistency to fluids. THERAPEUTIC USE: Analgesic, Anesthetic Adjunct, Opioid DOSE: Adult - dosage should be individualized, General anesthesia primary anesthetic agent, 8-30 mcg/kg IV with 100% oxygen and a muscle relaxant, then 0.5-10 mcg/kg as needed in response to signs of lightening of anesthesia; MAX 30 mcg/kg/procedure; analgesic adjunct to balanced general anesthesia, 1-8 mcg/kg IV (approximately 1 mcg/kg/hr of estimated surgical duration); 75% given prior to intubation, then incrementally as 10-50 mcg IV as needed in response to signs of lightening of analgesia. Pedia - General anesthesia, cardiovascular surgery (age under 12 yr) 10-25 mcg/kg with 100% oxygen, additional doses up to 25-50 mcg for maintenance of anesthesia CONTRA-INDICATIONS: hypersensitivity to sufentanil, intolerance to opioid agonists ADVERSE EFFECTS: Anaphylaxis, Cardiac arrest, Hypertension, Muscle rigidity, Respiratory depression THERAPEUTIC USE: Antiacne Antibacterial, Antibiotic, Antiseborrheic, Dermatological Agent, Sulfonamide DOSE: Adult – Bacterial infection of eye, Superficial infections including conjunctivitis a)ophthalmic solution, 1-2 drops into conjunctival sac(s) of affected eye(s) every 2-3 hr initially; taper by increasing time intervals as condition responds; usual duration 7-10 days. Pedia – Bacterial infection of eye, Superficial infections including conjunctivitis a)(2 months of age and older) ophthalmic solution, 1-2 drops into conjunctival sac(s) of affected eye(s) every 2-3 hr initially; taper by increasing time intervals as condition responds; usual duration 7-10 days CONTRA-INDICATIONS: hypersensitivity to sulfonamide products Sulpiride Dogmatil Genprid Capsule 50mg THERAPEUTIC USE: Dopamine Antagonist DOSE: Adult – oral dose of sulpiride in the treatment of Schizophrenia is 200 to 400mg twice daily, with gradual increases based on clinical response to a max. of 1200 mg daily CONTRA-INDICATIONS: Hypersensitivity to sulpiride, Pheochromocytoma, Parkinson's disease Sulprostone Nalador Injection 500mcg THERAPEUTIC USE: Prostaglandin DOSE: Adult – Cervical ripening procedure, Intracervical application of 50 to 100 mcg of sulprostone gel has been used to induce cervical priming prior to first-trimester abortion. The drug was given 6 to 8 hours prior to curettage 65 PRODUCT DESCRIPTION TRADE NAME DOSAGE FORM/STRENGTH PRODUCT INFORMATION CONTRA-INDICATIONS: Hypersensitivity to sulprostone, Acute pelvic inflammatory disease, Uterine scars Suxamethonium Chloride Anectine Midarine Injection 100mg/2mL Tamoxifen Citrate Nolvadex Tablet 10mg Teicoplanin Targocid Injection 200mg Injection 10,000units Tenecteplase Terbinafine HCl Lamifen Lamisil Injection 40iu/0.5mL Tetanus Toxoid (Purified) Tetracosactrin Tablet 250mg Synacthen Inj. 250mcg/mL Inj. 1mg/mL THERAPEUTIC USE: Musculoskeletal Agent, Neuromuscular Blocker, Depolarizing, Skeletal Muscle Relaxant DOSE: Adult – Induction of neuromuscular blockade, Adjunct to general anesthesia, to facilitate endotracheal intubation, and to provide skeletal muscle relaxation during surgery or mechanical ventilation a)short procedures, 0.6 mg/kg IV (range 0.3 to 1.1 mg/kg) over 10 to 30 seconds b)long procedures, 2.5 to 4.3 mg/min continuous IV infusion c)long procedures, 0.3 to 1.1 mg/kg IV initially followed by 0.04 to 0.07 mg/kg at appropriate intervals d)3 to 4 mg/kg IM, if suitable vein is inaccessible; MAX 150 mg. Pedia – emergency tracheal intubation, infants and small pediatric patients, 2 mg/kg IV CONTRA-INDICATIONS: acute phase of major trauma/major burns, extensive denervation of skeletal muscle, hypersensitivity to succinylcholine, personal or familial history of malignant hyperthermia, skeletal muscle myopathies, upper motor neuron injury ADVERSE EFFECTS: Apnea, Bradyarrhythmia, Especially in children, Cardiac arrest Cardiac dysrhythmia, Especially in children, Hyperkalemia, Immune hypersensitivity reaction, Malignant hyperthermia, Prolonged neuromuscular block, Respiratory depression, Rhabdomyolysis, With myoglobinemia, in children, Tachyarrhythmia THERAPEUTIC USE: Antiestrogen DOSE: Adult – Adjuvant treatment for breast cancer, 20 to 40 mg ORALLY daily for 5 years. Pedia – safety and efficacy beyond one year of therapy has not been established in girls aged 2 to 10 years treated for McCune-Albright Syndrome and precocious puberty CONTRA-INDICATIONS: concomitant coumarin-type anticoagulant therapy; deep vein thrombosis, hypersensitivity to tamoxifen citrate or any component of the product, pulmonary embolus ADVERSE EFFECTS: Cataract surgery, Cerebrovascular accident, Malignant neoplasm of endometrium of corpus uteri, Pulmonary embolism, Sarcoma of uterus THERAPEUTIC USE: Antibiotic, Glycopeptide DOSE: Adult – IM and IV in clinical trials in loading doses of 12 mg/kg, with 3 to 6 mg/kg/day as a single daily maintenance dose. The loading dose can be given as 2 doses at 12 hour intervals. Alternatively, 400 to 600 mg of IV teicoplanin can be given as a single loading dose, and followed with 200 to 600 mg either IV or IM once daily for maintenance therapy. Pedia – IV was effective and well tolerated when dosed at 3 to 6 mg/kg/day in children 2 to 12 years old for the treatment of gram-positive infections CONTRA-INDICATIONS: Hypersensitivity to teicoplanin ADVERSE EFFECTS: Hypersensitivity reaction THERAPEUTIC USE: Thrombolytic, Tissue Plasminogen Activator DOSE: Adult – Acute myocardial infarction, less than 60 kg, give 30 mg; 60 to 69 kg, give 35 mg; 70 to 79 kg, give 40 mg; 80 to 89 kg, give 45 mg; 90 kg or above, give 50 mg; administer single IV bolus over 5 seconds. Pedia - safety and efficacy have not been established in pediatric patients CONTRA-INDICATIONS: active internal bleeding, hypersensitivity to tenecteplase, history of cerebrovascular accident, intracranial or intraspinal surgery or trauma within 2 months intracranial neoplasm, arteriovenous malformation, or aneurysm, known bleeding diathesis, severe uncontrolled hypertension, intracranial aneurysm ADVERSE EFFECTS: Anaphylaxis, Bleeding, Major, Cardiac dysrhythmia, Reperfusion, Cerebrovascular accident, Cholesterol embolus syndrome, Immune hypersensitivity reaction, Intracranial hemorrhage THERAPEUTIC USE: Allylamine, Antifungal DOSE: Adult – Onychomycosis due to dermatophyte; ORAL, 250 mg daily for 6 wk for fingernails; 12 wk for toenails. Pedia – Tinea capitis, less than 25 kg, 125 mg/day ORALLY; 25 to 35 kg, 187.5 mg/day ORALLY; greater than 35 kg, 250 mg/day ORALLY CONTRA-INDICATIONS: hypersensitivity to terbinafine products ADVERSE EFFECTS: Agranulocytosis, Cutaneous lupus erythematosus, Erythema multiforme, Liver failure, Neutropenic disorder, Pancytopenia, Stevens-Johnson syndrome, Systemic lupus erythematosus, Toxic epidermal necrolysis THERAPEUTIC USE: Toxoid DOSE: Adult – Tetanus; Prophylaxis, 0.5 mL IM x 3 doses given at 0 and 4-8 weeks, and at 6-12 months after second dose; booster dose every 10 years. Pedia – Not for use in children less than 7 years of age. Tetanus; Prophylaxis, (7 years and older) 0.5 mL IM x 3 doses given at 0 and 4-8 weeks, and at 6-12 months after second dose; booster dose every 10 years CONTRA-INDICATIONS: febrile illness or acute infection; defer vaccination, history of systemic allergic or neurologic reactions to previous injection of the vaccine; only passive immunization should be given using Tetanus Immune Globulin (Human) (TIG), hypersensitivity to any component of the vaccine, including thimerosal ADVERSE EFFECTS: Anaphylaxis, Arthus type urticaria THERAPEUTIC USE: Diagnostic Agent, Adrenocortical Function, Endocrine-Metabolic Agent, Pituitary Hormone, Anterior DOSE: Adult – Adrenal insufficiency, Standard-dose; Diagnosis a)0.25 to 0.75 mg IM or IV over 2 min, diluted in NS b)IV infusion 0.04 mg/h 66 PRODUCT DESCRIPTION TRADE NAME DOSAGE FORM/STRENGTH Synacthen Depot Tetracycline Theophylline Hostacycline Laticyn Teracin Eye Ointment 1% Ointment 3% Tablet 250mg Quibron-T/SR Tablet 300mg Theoped Thioguanine Lanvis Tablet 40mg Thiopentone Sodium (Thiopental) Intraval Injection 500mg Thrombin Tianeptine Tigecycline Topical 5000iu Stablon Tygacil Tablet 12.5mg Injection 50mg PRODUCT INFORMATION over 6 h, diluted in glucose or saline solutions. Pedia – Adrenal insufficiency, Standard-dose; Diagnosis a)(2 y of age or less) 0.125 mg IM or IV over 2 min, diluted in NS b)(over 2 y) 0.25 to 0.75 mg IM or IV over 2 min, diluted in NS CONTRA-INDICATIONS: history of hypersensitivity to cosyntropin ADVERSE EFFECTS: Immune hypersensitivity reaction, Pancreatitis THERAPEUTIC USE: Amebicide, Intestinal, Antiacne, Antibacterial, Antibiotic, Antiprotozoal, Antiulcer, Helicobacter Pylori DOSE: Adult – Amebic infection, 1 g/day ORALLY divided in 2 to 4 equal doses; severe, 500 mg ORALLY 4 times daily. Pedia – Amebic infection (older than 8 years) 25 to 50 mg/kg/day ORALLY divided in 4 equal doses CONTRA-INDICATIONS: hypersensitivity to tetracycline products, last half of pregnancy/infancy/childhood up to 8 years of age ADVERSE EFFECTS: Bulging fontanelle THERAPEUTIC USE: Bronchodilator, Methylxanthine DOSE: Adult – extended-release 12 h formulation, initial, 300 mg/day ORALLY divided every 12 h; after 3 days if tolerated, 400 mg/day ORALLY divided every 12 h; after 3 more days if tolerated, 600 mg/day ORALLY divided every 12 h. Pedia – extended-release 12 h formulation, less than 45 kg, initial, 12 to 14 mg/kg/day (MAX 300 mg/day) ORALLY divided every 12 h; after 3 days if tolerated, 16 mg/kg/day (MAX 400 mg/day) ORALLY divided every 12 h; after 3 more days if tolerated, 20 mg/kg/day (MAX 600 mg/day) ORALLY divided every 12 hr. CONTRA-INDICATIONS: hypersensitivity to theophylline, or to any product component, known allergy to corn or corn products; in dextrose containing solutions. ADVERSE EFFECTS: Atrial fibrillation, Intracranial hemorrhage, Seizure, Stevens-Johnson syndrome, Tachyarrhythmia THERAPEUTIC USE: Antimetabolite, Antineoplastic Agent DOSE: Adult – Acute myeloid leukemia, Induction and consolidation therapy only, single agent, 2 mg/kg/day ORALLY; after 4 wk may increase to 3 mg/kg/day if no improvement or myelosuppression; adjust accordingly for combination therapy. Pedia – Acute myeloid leukemia, Induction and consolidation therapy only, single agent, 2 mg/kg/day ORALLY; after 4 wk may increase to 3 mg/kg/day if no improvement or myelosuppression; adjust accordingly for combination therapy CONTRA-INDICATIONS: Patients whose disease has demonstrated prior resistance to thioguanine or mercaptopurine ADVERSE EFFECTS: Gastrointestinal necrosis, With combination regimens, Hepatotoxicity, Hyperuricemia, Infectious disease, Jaundice, Liver function tests abnormal, Myelosuppression, Perforation of intestine THERAPEUTIC USE: Anesthetic Adjunct, Barbiturate, Short Acting DOSE: Adult – General anesthesia, slow induction, 50 to 75 mg (2 to 3 mL of 2.5% solution) slow IV, single dose at 20 to 40 sec intervals; rapid induction, 210 to 280 mg (3 to 4 mg/kg) IV divided in 2 to 4 doses; maintenance, 25 to 50 mg IV repeated as needed or continuous IV infusion of 0.2% or 0.4% solution. Pedia – not FDA approved for use in pediatric patients. General anesthesia a)induction, (neonate) 3 to 4 mg/kg; (1 to 6 month) 5 to 8 mg/kg IV; (1 to 15 y) 5 to 6 mg/kg IV b)(age 1 to 15 y) maintenance, intermittent IV 1mg/kg as needed CONTRA-INDICATIONS: absence of suitable veins for intravenous administration, acute intermittent porphyria or variegate porphyria, hypersensitivity to thiopental products/barbiturate ADVERSE EFFECTS: Anaphylaxis, Apnea, Finding of intracranial pressure, Hemolytic anemia, Laryngeal spasm, Myocardial dysfunction, Radial neuropathy, Respiratory depression THERAPEUTIC USE: Hemostatic DOSE: Adult - Control of hemorrhage, mild/moderate, apply 100 units/mL TOPICALLY as needed; severe, apply 1000 units/mL TOPICALLY as needed. Pedia - safety and efficacy in children have not been established CONTRA-INDICATIONS: hypersensitivity to thrombin or components of bovine origin, direct circulatory injection; extensive intravascular clotting and even death may occur. ADVERSE EFFECTS: Antibody development THERAPEUTIC USE: Antidepressant, Tricyclic, Dibenzothiazepine DOSE: Adult – Depression, 25 to 50mg daily effective (usually 3 divided doses; 2 or 4 times daily in some studies) CONTRA-INDICATIONS: Prior hypersensitivity to tianeptine THERAPEUTIC USE: Antibiotic, Glycylcycline DOSE: Adult – Infection of skin &/or subcutaneous tissue, Complicated, initial dose is 100 mg IV followed by 50 mg IV every 12 hours; duration is for 5 to 14 days depending on the severity and site of infection and clinical and bacteriological progress; Infectious disease of abdomen, Complicated, initial dose is 100 mg IV followed by 50 mg IV every 12 hours; duration is for 5 to 14 days depending on the severity and site of infection and clinical and bacteriological progress. Pedia – not recommended in patients under 18 years of age; safety and efficacy not established CONTRA-INDICATIONS: hypersensitivity to tigecycline ADVERSE EFFECTS: Pancreatitis, acute 67 PRODUCT DESCRIPTION Timolol Maleate TRADE NAME Apimol Ocumol DOSAGE FORM/STRENGTH Eye Drops 0.25% Eye Drops 0.5% Tinzaparin Sodium Innohep Injection 3500iu Injection 4500iu Inj. 14,000iu Inj. 20,000iu Tirofiban Aggrastat Injection 0.25mg/mL Tobramycin /Dexamethasone Tobradex Eye Ointment Tocopherol Evion Capsule 400mg Tablet 100mg Tolterodine Detrusitol Tablet 2mg Topiramate Topamax Tablet 25mg Tablet 100mg Tablet 200mg PRODUCT INFORMATION THERAPEUTIC USE: Antianginal, Antiglaucoma, Antihypertensive, Antimigraine, Beta-Adrenergic Blocker, Nonselective, Cardiovascular Agent DOSE: Adult – Open-angle glaucoma a)1 drop (0.25%-0.5%) in affected eye(s) once or twice daily b)1 drop (0.25%-0.5%) in affected eye(s) once daily c)1 drop (0.5% solution) in affected eye(s) once daily in the morning. Pedia – Open-angle glaucoma, 1 drop (0.25%-0.5% gelforming solution) in affected eye(s) once daily CONTRA-INDICATIONS: bronchial asthma or chronic obstructive pulmonary disease, cardiogenic shock, hypersensitivity to timolol, overt cardiac failure, second and third degree AV block, severe sinus bradycardia ADVERSE EFFECTS: Bronchospasm, Cardiac dysrhythmia, Myocardial infarction THERAPEUTIC USE: Anticoagulant, Low Molecular Weight Heparin DOSE: Adult – 100 international units antifactor Xa activity equals 1 mg tinzaparin sodium Deep venous thrombosis, In conjunction with warfarin, 175 international units anti-Xa/kg SC once daily for at least 6 days and until the patient is adequately anticoagulated with warfarin. Pedia – safety and efficacy in children have not been established CONTRA-INDICATIONS: active major bleeding, hypersensitivity to tinzaparin, heparin, sulfites, pork prod, history of heparin-induced thrombocytopenia, history of tinzaparin-induced thrombocytopenia ADVERSE EFFECTS: Anaphylaxis, Granulocytopenic disorder, Hemorrhage, Major, Pancytopenia, Priapism, Thrombocytopenia THERAPEUTIC USE: Glycoprotein Iib/IIIa Inhibitor, Platelet Aggregation Inhibitor DOSE: Adult – Acute coronary syndrome, 0.4 mcg/kg/min IV for 30 min then 0.1 mcg/kg/min for 12-24 hr after angioplasty/atherectomy. Pedia – safety and efficacy in pediatric patients have not been established CONTRA-INDICATIONS: aortic dissection, bleeding, major surgery, trauma, stroke within 30 days, concomitant use of another parenteral GPIIb/IIIa inhibitor, hemorrhagic stroke, history of intracranial hemorrhage, neoplasm, arteriovenous malformation, or aneurysm, hypersensitivity to tirofiban or other product components, pericarditis, severe hypertension, thrombocytopenia following prior tirofiban administration ADVERSE EFFECTS: Bleeding, Major, Dissecting aneurysm of coronary artery, Thrombocytopenia THERAPEUTIC USE: Aminoglycoside/Corticosteroid Combination DOSE: Adult – Bacterial infection of eye; Treatment and Prophylaxis – Inflammatory disorder of the eye, Steroid-responsive a)ophthalmic ointment, apply one-half inch ribbon OPHTHALMICALLY into conjunctival sac(s) 3 to 4 times daily b)ophthalmic suspension, initial, 1 to 2 drops OPHTHALMICALLY every 2 h for 24 to 48 h, then 1 to 2 drops every 4 to 6 h. Pedia – Bacterial infection of eye; Treatment and Prophylaxis – Inflammatory disorder of the eye, Steroid-responsive a)2 y and older, ophthalmic ointment, apply one-half inch ribbon OPHTHALMICALLY into conjunctival sac(s) 3 to 4 times daily b)2 y and older, ophthalmic suspension, initial, 1 to 2 drops OPHTHALMICALLY every 2 h for 24 to 48 h, then 1 to 2 drops every 4 to 6 h CONTRA-INDICATIONS: viral or mycobacterial infection of the eye, fungal diseases of the eye, hypersensitivity to tobramycin or dexamethasone ADVERSE EFFECTS: Glaucoma, Raised intraocular pressure, Secondary infection THERAPEUTIC USE: Colorectal Agent, Emollient, Nutritive Agent, Protectant, Dermatological, Vitamin E (class) DOSE: Adult – Vitamin E deficiency is 4 to 5 times the Recommended Dietary Allowance (RDA) (15 milligrams/day for adults). Doses as high as 300 milligrams may be necessary. Pedia – Vitamin E deficiency a)Premature or low-birth-weight neonates: vitamin E 25 to 50 units/day results in normal plasma tocopherol levels in 1 week b)Children with malabsorption syndrome: water-miscible vitamin E 1 unit/kilogram/day (to raise plasma tocopherol to within the normal range within 2 months and to maintain normal plasma concentrations) CONTRA-INDICATIONS: Intravenous use in infants THERAPEUTIC USE: Antimuscarinic, Urinary Antispasmodic DOSE: Adult – Bladder muscle dysfunction – overactive, With symptoms of urinary frequency, urgency, or urge incontinence a)2 mg ORALLY twice daily (immediate-release); may adjust dose to 1 mg twice daily (immediate-release) depending on tolerability and response b)4 mg ORALLY once daily (extended-release); may adjust dose to 2 mg once daily (extended-release) depending on tolerability and response. Pedia – safety and effectiveness in pediatric patients has not been established CONTRA-INDICATIONS: gastric retention, hypersensitivity to tolterodine products, uncontrolled narrow-angle glaucoma, urinary retention ADVERSE EFFECTS: Anaphylactoid reaction, Angioedema, Dementia, Memory impairment THERAPEUTIC USE: Anticonvulsant, Fructopyranose Sulfamate DOSE: Adult & Pedia – Partial seizure, Initial monotherapy, 1st week, 25 mg ORALLY twice daily (morning and evening); 2nd week, 50 mg ORALLY twice daily; 3rd week, 75 mg ORALLY twice daily; 4th week, 100 mg ORALLY twice daily; 5th week, 150 mg ORALLY twice daily; 6th week (maximum dose) 200 mg ORALLY twice daily. Pedia – Efficacy in seizures not established in children under 2 years of age. Pedia – Partial seizure, Initial monotherapy CONTRA-INDICATIONS: Hypersensitivity to topiramate 68 PRODUCT DESCRIPTION Trace Elements TRADE NAME Peditrace Tracutil Tragacanth Injection Adult Injection Pedia Powder Tramadol HCl Tramal Tranexamic Acid Cyklokapron Tretinoin DOSAGE FORM/STRENGTH Caspule 50mg Inj. 100mg/2mL SR Tablet 100mg Inj. 100mg/mL Tablet 500mg Acretine Retin-A Cream 0.05% Triamcinolone Acetonide Kenacort Injection 40mg/mL Trihexyphenidyl HCl Artane Tablet 2mg Tablet 5mg PRODUCT INFORMATION ADVERSE EFFECTS: Anemia, Body temperature above normal, Dyspnea, Hepatitis, Hyperammonemia, With or without encephalopathy, Hyperchloremia, Hypohidrosis, Leukopenia, Liver failure, Metabolic acidosis, normal anion gap (NAG),Nephrolithiasis Pancreatitis, Renal tubular acidosis THERAPEUTIC USE: Intended to meet the basal requirement of trace elements during IV nutrition of Adults, children and infants. DOSE: Adult – recommended daily dose in patients with basal requirements is 10mL (1ampoule), moderately increased requirements the daily dose may be up to 20mL (2amp.) accompanied by monitoring of the trace element status. Pedia – 1mL/Kg/Day to a max. dose of 15mL CONTRA-INDICATIONS: Wilson's disease THERAPEUTIC USE: Suspending agents in lotions, mixtures and extemporaneous preparations and prescriptions. It is used with emulsifying agents largely to increase consistency and retard creaming. It is sometimes used as a demulcent in sore throat and the jelly-like product formed when the gum is allowed to swell in water serves as a basis for pharmaceutical jellies, also used in various confectionery products and as a pill excipient. THERAPEUTIC USE: Analgesic, Opioid DOSE: Adult - Pain, Moderate to moderately severe a)(immediate-release) titration schedule, 25 mg/day ORALLY every morning, titrated in 25 mg increments as separate doses every 3 days to reach 25 mg 4 times daily; then, may increase total daily dose by 50 mg as tolerated to reach 50 mg 4 times daily; after titration, 50 to 100 mg ORALLY every 4-6 as needed can be used; MAX 400 mg/day b)(immediate-release) rapid analgesic onset, 50 to 100 mg ORALLY every 4-6 h; MAX 400 mg/day c)(extended-release) 100 mg ORALLY once daily, may titrate up by 100-mg increments every 5 days if necessary; MAX 300 mg/day. Pedia - safety and efficacy in patients under 16 years of age has not been established a dosage of 50 to 100 milligrams (mg) every 4 to 6 hours can be administered as needed, to a maximum of 400 mg/day CONTRA-INDICATIONS: hypersensitivity to tramadol, any other components of the product, or opioids, intoxication with alcohol, hypnotics, narcotics, centrally acting analgesics, opioids, or psychotropic drugs; may worsen central nervous system and respiratory depression ADVERSE EFFECTS: Anaphylactoid reaction, Dyspnea, Hallucinations, Impaired cognition, Orthostatic hypotension, Seizure, Syncope, Tachyarrhythmia THERAPEUTIC USE: Hemostatic DOSE: Adult - Gastrointestinal hemorrhage, 1 gm IV every 4-6 hours for 2-3 days followed by 1.5 gm ORALLY 3-4 times a day for 3-7 days. Pedia - Hemophilia - Hemorrhage; Prophylaxis - Tooth extraction; pre-op, 10 mg/kg IV as a single dose with replacement therapy immediately prior to extraction or 25 mg/kg ORALLY 3-4 times a day beginning one day before surgery CONTRA-INDICATIONS: active intravascular clotting process, acquired defective color vision, subarachnoid hemorrhage, hypersensitivity to tranexamic acid ADVERSE EFFECTS: Thrombotic disorder THERAPEUTIC USE: Antiacne, Antineoplastic Agent, Dermatological Agent, Retinoid DOSE: Adult - Acne vulgaris, apply 0.025%-0.1% cream or 0.05% LIQ topically at bedtime; Hyperpigmentation of skin, Facial mottling; with comprehensive skin care and sunlight avoidance programs; Adjunct - apply 0.05% cream at bedtime. Pedia - Acute promyelocytic leukemia, FAB M3, Induction therapy in patients who are refractory to, or have relapsed from, anthracycline chemotherapy, 1 y and older, 45 mg/m(2)/day ORALLY (divided twice daily) for 90 days or 30 days past complete remission whichever comes first CONTRA-INDICATIONS: hypersensitivity to tretinoin or parabens ADVERSE EFFECTS: Disorder of skin pigmentation, Topical, Dyspnea, Fever, Leukocytosis, Oral, Pericardial effusion, Pleural effusion, Pseudotumor cerebri, Oral; especially pediatrics, Pulmonary infiltrate, Retinoid adverse reaction, Headache, N/V, fever, skin dryness, bone pain, Weight gain THERAPEUTIC USE: Adrenal Glucocorticoid, Anti-Inflammatory, Corticosteroid, Intermediate, Dental Agent, Endocrine-Metabolic Agent, Immune Suppressant DOSE: Adult - Acquired hemolytic anemia, IM, initial, 2.5 to 60 mg/day injected deeply into the gluteal muscle, doses can be adjusted between 20 to 80 mg/day as needed. Pedia - Acquired hemolytic anemia, 6 to 12 y, IM, initial, 40 mg/day injected deeply into the gluteal muscle, dose may vary upon severity of symptoms CONTRA-INDICATIONS: hypersensitivity to triamcinolone acetonide or any other ingredients in the preparation, local viral, fungal, or bacterial infections, status asthmaticus, acute asthma ADVERSE EFFECTS: Cataract, Glaucoma, Hyperglycemia, Primary adrenocortical insufficiency, Pulmonary tuberculosis THERAPEUTIC USE: Anticholinergic, Antiparkinsonian DOSE: Adult - Parkinson's disease, initial, 1 mg/day ORALLY on first day, then increase in 2 mg increments at 3 to 5 day intervals; 6 to 10 mg normal daily dose; 12 to 15 mg/day postencephalitic; maintenance, 5 to 15 mg ORALLY DAILY (divided 3 to 4 times daily). Pedia - safety and efficacy have not been established in pediatric patients 69 PRODUCT DESCRIPTION TRADE NAME DOSAGE FORM/STRENGTH Triprolidine/Pseudoephedrine Actifed Flucare Sedofan Tropicamide Mydriacyl Eye Drops 0.5% Eye Drops 1% Eye Minims 0.5% Tropisetron Navoban Capsule 5mg Inj. 5mg/5mL Injection 2iu Injection 10iu Tuberculin Ursodeoxycholic Acid Vancomycin Varicella Zoster Virus Vaccine Syrup Urdox Tablet 300mg Ursofalk Vancoled Injection 500mg Vancolon Varilrix Injection PRODUCT INFORMATION CONTRA-INDICATIONS: glaucoma, narrow-angle, hypersensitivity to trihexyphenidyl or any of tablet or elixir ingredients, under 3 years of age, tardive dyskinesias ADVERSE EFFECTS: Angle-closure glaucoma, Disorientated, Raised intraocular pressure THERAPEUTIC USE: For the symptomatioc relief of upper respiratory tract disorders which are benefited by a combination of a histamine receptor antagonist and a nasal decongestant, includes allergic rhinitis, vasomotor rhinitis, common cold and influenza. DOSE: Adult – 10mL three times daily. Pedia (6 -12 years) 5mL three times daily; (2 to 5years) – 2.5mL three times daily; (6months – under 2years) 1.25mL three times daily. CONTRA-INDICATIONS: Severe hypertension, monoamine oxidase inhibitors, antibacterial agent Furazolidone, exhibited intolerance to it or to any of its constituents. ADEVERSE EFFECTS: CNS depression, drowsiness, sleep disturbances, skin rashes, tachycardia, dryness of the mouth, urinary retention. THERAPEUTIC USE: Antimuscarinic, Mydriatic-Cycloplegic DOSE: Adult - Cycloplegia - Mydriasis induction a)examination of fundus, 1 to 2 drops 0.5% solution in the eye(s) 15 to 20 min prior to exam b)refractive procedures, 1 to 2 drops 1% solution in the eye(s), may be repeated in 5 min. Pedia - Cycloplegia - Mydriasis induction a)examination of fundus, 1 to 2 drops of 0.5% solution in the eye(s) 15 to 20 min prior to exam b)refractive procedures, 1 to 2 drops of 0.5% or 1% solution in the eye(s), may be repeated in 5 min CONTRA-INDICATIONS: primary glaucoma or a tendency toward glaucoma, sensitivity to tropicamide ADVERSE EFFECTS: Abnormal behavior, Systemic absorption, Cardiac arrest, Confusion, Increased heart rate, Light intolerance, Psychotic disorder, Raised intraocular pressure, Xerostomia THERAPEUTIC USE: Serotonin Receptor Antagonist, 5-HT3 DOSE: Adult - Chemotherapy-induced nausea and vomiting, 5 mg IV on the day of chemotherapy (15 minutes prior to chemotherapy regimens), followed by oral doses of 5 mg every 6 to 24 hours on subsequent days (for up to 7 days). Pedia -Postoperative nausea and vomiting; Prophylaxis, single dose of tropisetron (0.1mg/kg up to a maximum of 5 mg) was more effective than placebo in preventing postoperative nausea and vomiting in children using morphine patient-controlled analgesia (PCA). After induction of anesthesia, tropisetron was given intravenously. At the end of surgery. CONTRA-INDICATIONS: Hypersensitivity to tropisetron THERAPEUTIC USE: Diagnostic Agent, Tuberculin DOSE: Adult - Tuberculosis; Diagnosis, Mantoux test, inject 0.1 mL (5 tuberculin units) INTRADERMALLY. Pedia - Tuberculosis; Diagnosis, Mantoux test, inject 0.1 mL (5 tuberculin units) INTRADERMALLY CONTRA-INDICATIONS: hypersensitivity to tuberculin purified protein derivative, previous severe reaction (eg, vesiculation, ulceration, necrosis) ADVERSE EFFECTS: Injection site disorder, Vesiculation, Necrosis, Skin ulcer THERAPEUTIC USE: Gastrointestinal Agent DOSE: Adult - Chemodissolution of bile duct stone, 8 to 10 mg/kg/day ORALLY divided into 2 to 3 doses; Gallstone, During rapid weight loss; Prophylaxis, 300 mg. Pedia - safety and effectiveness pediatric patients have not been established CONTRA-INDICATIONS: hypersensitivity to bile acids or ursodiol, calcified cholesterol stones, radiopaque stones, radiolucent bile pigment stones, compelling reason for cholecystectomy THERAPEUTIC USE: Antibiotic, Glycopeptide DOSE: Adult - Staphylococcal enterocolitis, 500mg to 2g orally daily divided every 6 to 8 hours for 7 to 10 days; Staphylococcal infectious disease, methicillin-resistant, 2 grams/day IV divided every 6-12 hr administered over at least 60 minutes. Pedia - Staphylococcal enterocolitis, 40mg/kg/day orally divided every 6 to 8 hr for 7 to 10 days; max. is 2 g daily; Staphylococcal infectious disease, methicillinresistant, 10mg/kg IV every 6 hr administered over at least 60 min.; max. 2 g/day; infants and neonates, initial, 15 mg/kg IV, followed by 10 mg/kg IV every 12 hr (administered over at least 60 min.) for neonates in the 1st week of life, and every 8hr from the second to 4th week of life CONTRA-INDICATIONS: hypersensitivity to vancomycin products, solutions containing dextrose (pre-mixed solutions in GALAXY plastic container); may be contraindicated in patients with known corn or corn product allergies ADVERSE EFFECTS: Anaphylaxis, Nephrotoxicity, Neutropenia, Ototoxicity, Thrombocytopenia THERAPEUTIC USE: Vaccine DOSE: Adult - Varicella, Varivax(R); Prophylaxis, 0.5 mL SUBQ x 2 doses, at 0 and 4 to 8 wks. Pedia - safety and effectiveness in children below the age of 12 months have not been established. Varicella, Prophylaxis a)(13 y and older) 0.5 mL SUBQ x 2 doses, at 0 and 4 to 8 wks b)(12 mo to 12 y) 0.5 mL SUBQ; the ACIP recommends the first dose at 12 to 18 mo and a second dose at 4 to 6 y CONTRA-INDICATIONS: anaphylactoid/anaphylactic reaction to neomycin, anaphylactoid/anaphylactic reaction to gelatin, blood dyscrasias, cerebrospinal fluid leaks, concurrent immunosuppressive therapy, family history of congenital or hereditary immunodeficiency, unless the immune competence of the potential vaccine recipient is demonstrated, febrile illness, active including febrile respiratory illness, HIV- 70 PRODUCT DESCRIPTION Vasopressin Vecuronium Bromide Verapamil HCl TRADE NAME Lypressin DOSAGE FORM/STRENGTH Injection 20u/mL Pitressin Norcuron Isoptin Isoptin SR Injection 10mg Inj. 2.5mg/mL Syrup 50mg/mL Tablet 40mg Tablet 120mg Vinblastine Sulphate Velbe Injection 10mg/10mL Vincristine Sulfate Oncovin Inj. 1mg/mL Injection 2mg PRODUCT INFORMATION infection or AIDS, immunodeficiency states, history of primary or acquired; including cellular immune deficiencies, hypogammaglobulinemic and dysgammaglobulinemic states, and congenital immunodeficiency, lymphoma, leukemia, generalized malignancy, bone marrow malignancy or lymphatic system malignancy, pregnancy or may become pregnant within 3 months of receiving the vaccine, tuberculosis, active untreated ADVERSE EFFECTS: Anaphylaxis, Ataxia, Bell's palsy, Encephalitis, Exacerbation of asthma, Guillain-Barre syndrome, Polymyalgia rheumatica, Seizure, Non-febrile, Stevens-Johnson syndrome, Toxic epidermal necrolysis, Transverse myelopathy syndrome THERAPEUTIC USE: Endocrine-Metabolic Agent, GI Agent, Vasopressin (class) DOSE: Adult - Abdominal distension; Prophylaxis - Postoperative complication, initial, 5 units IM (0.25 mL) postoperatively; increase to 10 units (0.5 mL) at subsequent injections repeated at 3- or 4-hour intervals if necessary. Pedia - reduce dose proportionately for pediatric patients. Abdominal distension; Prophylaxis - Postoperative complication - for pediatric patients a proportionate reduction of the adult dose is recommended; adult dose: initial, 5 units IM (0.25 mL) postoperatively; increase to 10 units (0.5 mL) at subsequent injections repeated at 3- or 4-hour intervals if necessary. CONTRA-INDICATIONS: anaphylaxis or hypersensitivity to the drug or its components ADVERSE EFFECTS: Anaphylaxis, Cardiac arrest, Cardiac dysrhythmia, Coronary arteriosclerosis, Decreased cardiac output, Gangrenous disorder, Stenosis of bronchus, Water intoxication syndrome THERAPEUTIC USE: Musculoskeletal Agent, Neuromuscular Blocker, Non-Depolarizing DOSE: Adult - dosage must be individualized. Anesthesia, During surgery as an adjunct to general anesthesia to facilitate tracheal intubation or mechanical ventilation; Adjunct a)initial, 0.08-0.1 mg/kg IV bolus b)maintenance, 0.01-0.015 mg/kg IV 25-40 min after initial dose, repeat every 12-15 min as needed c)1 mcg/kg/min continuous IV infusion 20-40 min after initial intubation dose, after early evidence of spontaneous recovery; then adjust to maintain 90% suppression of twitch response; range 0.8-1.2 mcg/kg/min. Pedia - dosage must be individualized Safety and effectiveness not established in pediatric patients 7 weeks of age or younger. Anesthesia, During surgery as an adjunct to general anesthesia to facilitate tracheal intubation or mechanical ventilation; Adjunct a)(age 8 weeks-1 yr) more sensitive on a mg/kg basis than adults and recovery may take 1.5 times longer b)(age 1-10 yr) dosage must be individualized; may require slightly higher initial dose and slightly more frequent supplemental doses than adults c)(age 10-16 yr) initial, 0.08-0.1 mg/kg IV bolus d)(age 10-16 yr) maintenance, 0.01-0.015 mg/kg IV 25-40 min after initial dose, repeat every 12-15 min as needed; may require more frequent supplementation than adults CONTRA-INDICATIONS: hypersensitivity to vecuronium products ADVERSE EFFECTS: Anaphylaxis, Apnea, Bronchospasm, Hypotension, Muscle weakness Prolonged, Prolonged neuromuscular block, Tachyarrhythmia THERAPEUTIC USE: Antianginal, Antiarrhythmic, Group IV, Antihypertensive, Antimigraine, Calcium Channel Blocker, Cardiovascular Agent, Phenylalkylamine DOSE: Adult – Angina a)(extended-release) initial, 180 mg ORALLY once daily at bedtime; titrate to 480 mg at bedtime; MAX 540 mg at bedtime b)(immediate-release) 80 mg to 120 mg ORALLY three times daily. Pedia - oral formulations are not FDA approved in children under 18 yr. Atrial arrhythmia a)(less than 1 yr old) 0.1-0.2 mg/kg IV over 2 min, may repeat dose after 30 min b)(1-15 yr old) 0.1-0.3 mg/kg IV over 2 min (MAX 5 mg), may repeat dose after 30 min (MAX 10 mg) CONTRA-INDICATIONS: atrial fibrillation/flutter associated with accessory bypass tract, cardiogenic shock, congestive heart failure, second or third-degree atrioventricular block, hypersensitivity to verapamil/other calcium channel antagonists, sick sinus syndrome, symptomatic hypotension, ventricular tachycardia, wide-complex ADVERSE EFFECTS: Angina, Myocardial infarction, Syncope THERAPEUTIC USE: Antineoplastic Agent, Mitotic Inhibitor DOSE: Adult - Breast cancer, Unresponsive to endocrine surgery and hormonal therapy, first dose 3.7 mg/m(2) IV, second dose 5.5 mg/m(2) IV, third dose 7.4 mg/m(2) IV, fourth dose 9.25 mg/m(2) IV, fifth dose 11.1 mg/m(2) IV, at weekly intervals; increase dose weekly until MAX dose of 18.5 mg/m(2), target WBC count reduction to 3000 cells/mm(3); weekly maintenance dose 1 increment smaller than final initial dose. Pedia - Choriocarcinoma, Unresponsive to other chemotherapeutic agents, 3 mg/m(2) when used in combination chemotherapy regimens; dose modifications should be guided by hematologic tolerance CONTRA-INDICATIONS: bacterial infection, significant granulocytopenia, unless result of disease being treated ADVERSE EFFECTS: Azoospermia, Hypertension, Hyperuricemia, Leukopenia, Dose-limiting, Myelosuppression, Neurotoxicity THERAPEUTIC USE: Antineoplastic Agent, Mitotic Inhibitor DOSE: Adult - Acute leukemia, usual dose is 1.4 mg/m(2)/wk IV (MAX 2 mg; dose limit depends on patient, physician, protocol, and institution). Pedia - Acute leukemia a)10 kg or less, 0.05 mg/kg IV once weekly, varies per protocol b)over 10 kg, 1.5 to 2 mg/m(2) IV once weekly, varies per protocol. CONTRA-INDICATIONS: Charcot-Marie-Tooth syndrome, hypersensitivity to vincristine/products, intrathecal use ADVERSE EFFECTS: Myelosuppression, Neuromyopathy 71 PRODUCT DESCRIPTION Vitamin B Complex Vitamin B12 Warfarin Xylometazoline TRADE NAME Neurobion Tri - B DOSAGE FORM/STRENGTH Injection Tablet Cynovit Injection 1000mcg/mL Coumadin Tablet 1mg Tablet 2mg Tablet 2.5mg Tablet 3mg Tablet 5mg Tablet 10mg Decozal Otrivin Nasal Drops 0.05% Nasal Drops 0.1% Nasal Spray 0.1% Xylose Powder 5grams Zinc Oxide Ointment 10% Powder Zinc Sulphate Powder Syrup 4mg/mL PRODUCT INFORMATION THERAPEUTIC USE: Dietary supplements, Nutritional, B Vitamins Combinations DOSE: Adult - Oral 1 to 2 tablets three times daily. Ampoule – initial treatment 1amp daily by deep IM injection. After subsidence of the acute symptoms and in less severe cases, 2 or 3 ampoules weekly. ADVERSE EFFECTS: Rare hypersensitivity reactions THERAPEUTIC USE: Diagnostic Agent, Vitamin B12 Absorption, Nutritive Agent, Vitamin B DOSE: Adult – B12 deficiency a)normal absorption, 1000 mcg/day orally; if oral route not adequate, initial treatment same as for pernicious anemia; chronic treatment should be with oral B12 preparation b)malabsorption, 100 mcg IM or deep SC injection daily for 6 or 7 days; if clinical improvement and reticulocyte response, give same amount on alternate days for 7 doses; then every 3 to 4 days for another 2 to 3 wk; then 100 mcg monthly for life. Pedia - Cobalamin deficiency, 1000mcg/day orally, IM route is preferred; 30 to 50 mcg IM daily for 2 or more wk; then 100 mcg monthly to sustain remission; Pernicious anemia - 30 to 50mcg IM daily for 2 or more wk; then 100 mcg monthly to sustain remission CONTRA-INDICATIONS: hypersensitivity to cobalt, B12 products, or any component of the product ADVERSE EFFECTS: Anaphylaxis, Angioedema, Congestive heart failure, Peripheral vascular disease, Pulmonary edema THERAPEUTIC USE: Anticoagulant, Coumarin (class) DOSE: Adult - Atrial fibrillation - Thromboembolic disorder, initial, 2 to 5 mg orally/IV once a day; adjust dose based on the results of INR; usual maintenance, 2 to 10 mg orally/IV once a day. Pedia - safety and efficacy in pediatrics patients have not been established, although the use of warfarin for the treatment or prophylaxis of thrombosis is well established; more frequent INR determinations are recommended in pediatric patients CONTRA-INDICATIONS: abortion, eclampsia, preeclampsia, threatened, alcoholism; lack of patient cooperation, anesthesia, major regional or lumbar block, aneurysms; cerebral, dissecting aorta, bacterial endocarditis, bleeding tendencies of the gastrointestinal, genitourinary, or respiratory tract, blood dyscrasias, cerebrovascular hemorrhage, gastrointestinal, genitourinary or respiratory tract ulcerations or overt bleeding, hemorrhage, cerebrovascular, hemorrhagic tendencies, hypersensitivity to warfarin or any component of the product, inadequate laboratory facilities, malignant hypertension, pericarditis and pericardial effusion, pregnancy, known or suspected, psychosis; lack of patient cooperation, senility; lack of patient cooperation, spinal puncture and other procedures with potential for uncontrollable bleeding, surgery of central nervous system or eye, recent or potential, traumatic surgery resulting in large open surface, recent or potential ADVERSE EFFECTS: Cholesterol embolus syndrome, Hemorrhage, Hepatitis, Hypersensitivity reaction, Intraocular hemorrhage, Tissue necrosis THERAPEUTIC USE: Decongestant, Imidazoline DOSE: Adult – Rhinitis, for adults and children 12 years old or more, the recommended dose of xylometazoline 0.1% nasal solution is 2 to 3 drops in each nostril every 8 to 10 hours. The nasal spray (0.1%) is administered as 1 to 2 inhalations in each nostril every 8 to 10 hours. Should not be employed for longer than 3 to 5 days due to the risk of rebound congestion. Pedia – Rhinitis, In 2 to 12 years of age, 2 or 3 drops of xylometazoline 0.05% solution should be instilled in each nostril every 8 to 10 hours. Dropper dosage forms are preferred over sprays in children less than 6 years old since dosing can be more easily controlled; In children 6 months to 2 years of age, the usual pediatric dose of xylometazoline 0.05% solution is 2 to 3 drops in each nostril every 8 to 10 hours; Should not be employed for longer than 3 to 5 days to prevent the occurrence of rebound congestion CONTRA-INDICATIONS: Hypersensitivity to Xylometazoline or other adrenergic agents, Narrow-angle glaucoma, Rhinitis sicca, Children under age 6 years THERAPEUTIC USE: Diagnosis for intestinal malabsorption and steatorrhea DOSE: Adult - 5 or 25g dose in 200 to 300 mL (maxi 500 mL) of water for evaluating malabsorptive syndromes. Pedia - 500 mg/kg as a 5 to 10% solution in water, or 14.5 grams/square meter body surface in a 10% aqueous solution; Maximum pediatric dosage is 25 grams CONTRA-INDICATIONS: Absolute contraindications and precautions to this agent have not been determined; no information found at the time of this review ADVERSE EFFECTS: Nause, Intestinal bloating, Borborygmi, Vomiting, Cramping, Abdominal discomfort & Diarrhea. THERAPEUTIC USE: Prevention and soothing relief of nappy rash and redness of the skin. It is also recommended for use in minor burns and wounds. DOSE: Apply it evenly at every nappy change, particularly to the folds of the skin. Whereas for minor burns or wounds, clean and dry the affected area, apply it evenly to the affected area as necessary. THERAPEUTIC USE: Antipruritic, Hemorrhoidal Agent, Parenteral Mineral-Trace Mineral, Protectant, Dermatological, Throat Agent, Zinc Supplement DOSE: Adult - Zinc deficiency, 25 to 50 mg elemental zinc orally per day. Pedia - Zinc deficiency, 0.5 to 1 mg elemental zinc/kg orally daily, divided 1 to 3 times/day 72 PRODUCT DESCRIPTION Zoledronic Acid TRADE NAME Zometa DOSAGE FORM/STRENGTH Injection 4mg PRODUCT INFORMATION THERAPEUTIC USE: Bisphosphonate , Calcium Regulator DOSE: Adult - Bone metastasis - 4mg IV infused over 15min every 3-4 wk; administer with an oral calcium supplement of 500mg and a multiple vitamin containing 400 IU of vitamin D. Pedia - safety and effectiveness not established in children CONTRA-INDICATIONS: hypersensitivity to Zoledronic Acid or any of its excipients, hypocalcemia ADVERSE EFFECTS: Aseptic necrosis of bone, of the jaw, Hyperkalemia, Hypocalcemia, Nephrotoxicity, Pleural effusion 73