F170-120-01C Pathology Collaboration Agreement V03
advertisement

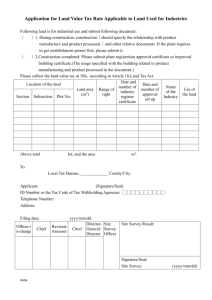
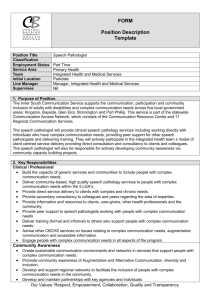
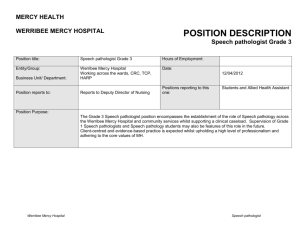
Pathology Collaboration Agreement Approved by: Yvonne Myal Signature on file Effective Date: 19-JUL-2011 Document # F170-120-01C Version # 03 Source Document: Research Policy 170-120-01 Research Study Title: Sponsor Protocol Number (as provided by Principal Investiigator) : Disease Site Group: Number of Cases Expected: Deliverables Requested: ____________________________ Signature of Principal Investigator _________________________ Printed Name ______________ Date (dd/mm/yyyy) Note: Pathologist’s time will be billed at a minimum charge of $35/10 minute interval. Collaborating Pathologist Statement of Responsibilities: As the Collaborating Pathologist for the above named clinical trial or research project, I am aware of the tissue requirements outlined in this protocol, agree to be the main pathology contact person in collaboration with the Principal Investigator, and will be responsible for the selection of tissue (blocks/slides) to be released. Terms of Agreement (check applicable boxes and provide details): 1. Research as part of non-clinical workload 2. Research work in lieu of clinical workload, as approved by U of M Pathology Department Head 3. Time Estimate: / per case _______________________________ Signature of Collaborating Pathologist _________________________ Printed Name ______________ Date (dd/mm/yyyy) Approval from Pathology Medical Director (or designate): ___________________________________ Signature - Dr. John Gartner _____Dr. John Gartner_________ Printed Name ________________ Date (dd/mm/yyyy) Diagnostic Services of Manitoba’s vision is to provide a high-quality, state-of-the-art diagnostic system for Manitoba that is known for its excellent customer service and which is centrally managed, cost-effective and sustainable. Website www.dsmanitoba.ca Page 1 of 1