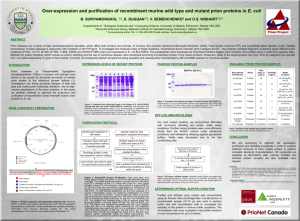
No Zn Refold for MMP
advertisement
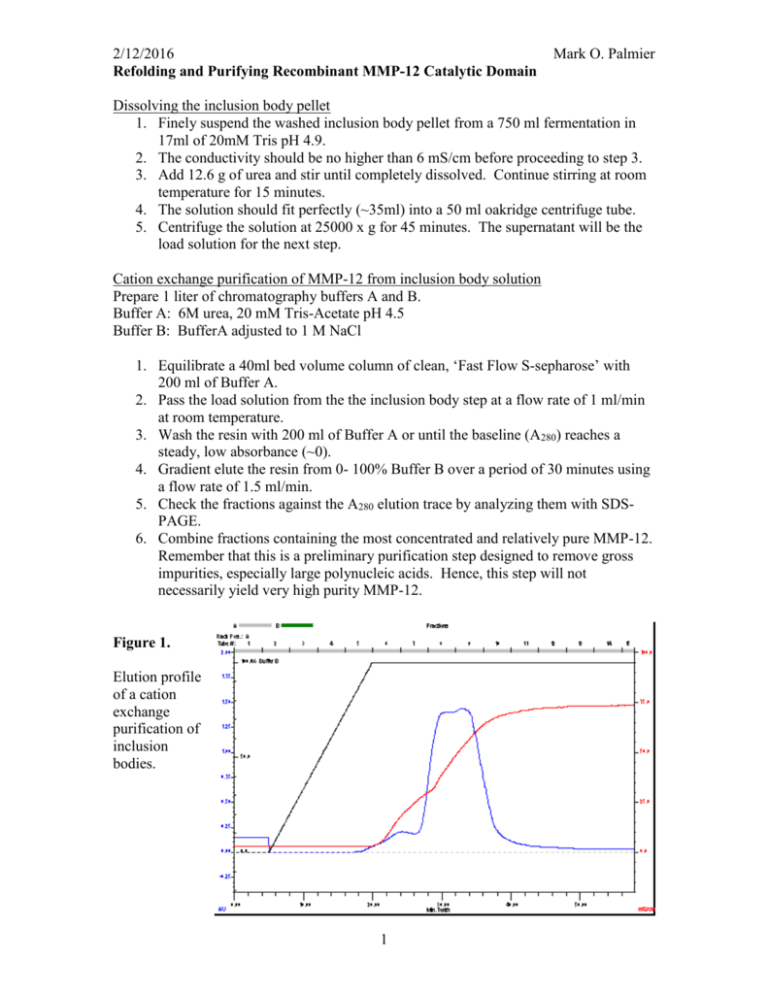
2/12/2016 Refolding and Purifying Recombinant MMP-12 Catalytic Domain Mark O. Palmier Dissolving the inclusion body pellet 1. Finely suspend the washed inclusion body pellet from a 750 ml fermentation in 17ml of 20mM Tris pH 4.9. 2. The conductivity should be no higher than 6 mS/cm before proceeding to step 3. 3. Add 12.6 g of urea and stir until completely dissolved. Continue stirring at room temperature for 15 minutes. 4. The solution should fit perfectly (~35ml) into a 50 ml oakridge centrifuge tube. 5. Centrifuge the solution at 25000 x g for 45 minutes. The supernatant will be the load solution for the next step. Cation exchange purification of MMP-12 from inclusion body solution Prepare 1 liter of chromatography buffers A and B. Buffer A: 6M urea, 20 mM Tris-Acetate pH 4.5 Buffer B: BufferA adjusted to 1 M NaCl 1. Equilibrate a 40ml bed volume column of clean, ‘Fast Flow S-sepharose’ with 200 ml of Buffer A. 2. Pass the load solution from the the inclusion body step at a flow rate of 1 ml/min at room temperature. 3. Wash the resin with 200 ml of Buffer A or until the baseline (A280) reaches a steady, low absorbance (~0). 4. Gradient elute the resin from 0- 100% Buffer B over a period of 30 minutes using a flow rate of 1.5 ml/min. 5. Check the fractions against the A280 elution trace by analyzing them with SDSPAGE. 6. Combine fractions containing the most concentrated and relatively pure MMP-12. Remember that this is a preliminary purification step designed to remove gross impurities, especially large polynucleic acids. Hence, this step will not necessarily yield very high purity MMP-12. Figure 1. Elution profile of a cation exchange purification of inclusion bodies. 1 2/12/2016 Mark O. Palmier Refolding purified denatured MMP-12 Prepare 1. Refolding buffer: 6 M urea, 20 mM Tris–HCl, 5 mM CaCl2, and 100 mM NaCl, pH 7.5. 2. Combine chosen fractions from the s-sepharose purification, dilute with refolding buffer to a protein concentration of 0.1 mg/ml. The resultant diluted protein is dialyzed in following steps: 1. Dialyze against refolding buffer containing 3, 1, and 0 M urea successively with each dialysis performed at 4ºC for 4 h against 5 volumes of the dialysis buffer. 2. Dialyze against 20 mM Tris–HCl, 5 mM CaCl2, pH 7.3 at 4ºC for 4 hours. 3. Add 40 ml of clean, water-hydrated s-sepharose and rock for 10 minutes in the orbital shaker set at 10ºC. (Note: The resin is simply water hydrated with no buffer agent.) 4. Adjust the slurry to 100μM ZnCl2 and rock for 45 minutes. 5. Pour into an empty column and wash with 20 mM Tris–HCl, 5 mM CaCl2, 0.1 mM ZnCl2 pH 7.3 until the baseline is zero or flat after passing at least one resin volume of the buffer. This step and the following steps are performed at room temperature. 6. Elute the folded MMP-12 with a linear gradient of 0 – 1M NaCl over 30 minutes using a 1.5 ml/min flow rate. 7. Collect 8 ml fractions and analyze suitable fractions by SDS-PAGE. 8. Determine [MMP-12] in the high purity fractions by the BioRad protein assay. 9. Check the activity of the renatured MMP-12 by kinetic analysis with FS-6. 10. Adjust the final pool of MMP-12 to 50% glycerol w/v, freeze aliquots and store at -80ºC. Figure 2. Elution profile of a cation exchange purification of the refolded MMP-12. 2 2/12/2016 Mark O. Palmier This enzyme preparation should stay active for at least one year with no detectable loss in catalytic activity when assayed with FS-6. The specific activity of wt MMP-12 CD with FS-6 is 133800 M-1 s-1. If the specificity constant (kcat/Km) is known for the MMP-12 mutant then a simple progress curve measuring activity with an [FS-6] << Km will suffice to measure the active site concentration. If a kcat/Km value is not known then a full active site titration must be done with a suitable fast, tight binding inhibitor like galardin (GM6001). Data: Lot0313082052_Intensity Model: kcat_Km_v2 Weighting: y No weighting 1000000 900000 800000 Chi^2/DoF = 4575766.65813 R^2 = 0.99978 RFU 700000 125nM T205K H206D MMP-12, 4M FS-6 600000 From previous AST the -1 -1 kcat/Km = 118290 ± 298 M s 500000 400000 This activity measurement indicates an enzyme concentration of 121nM 300000 0 50 100 150 200 250 300 350 time (s) 3 400 Fx k Et B 921973 ±0 118290 ±0 1.2108E-7 330932.9 ±1.054E-10 ±0