Lopez, ViecaAnadonMEPS181206
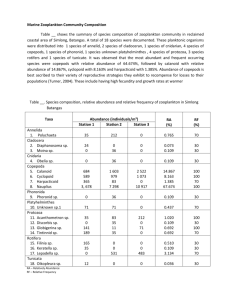
advertisement

1 Seasonal variation in the abundance and grazing rates of the first 2 stages of copepods in a temperate sea. 3 4 Running head: Copepods abundance and grazing rates 5 6 Eva López*, Leticia Viesca, Ricardo Anadón 7 Área de Ecología, Departamento de Biología de Organismos y Sistemas, Universidad de 8 Oviedo, C/ Catedrático Rodrigo Uría, s/n, CP 33071, Oviedo, Spain. 9 *E-mail address: evalop.uo@uniovi.es 10 11 12 Abstract: 13 The understanding of the role of copepods populations in oceanic ecosystems and carbon fluxes is limited 14 by the scarcity of information about their smallest size fraction and first developmental stages. Here, we 15 present an study that includes the whole copepod population, but with special emphasise on copepods < 16 200 µm and nauplii. Abundance of the different stages of copepods, and ingestion rates of nauplii, 17 copepods and copepodites belonging to the size fraction < 200 µm, were measured during an annual cycle 18 in three stations off Cudillero (Southern Bay of Biscay). Nauplii were the most abundant group in the 19 metazooplankton, with densities ranging between 1 - 48 individuals l-1. The highest abundances have 20 been found during late summer and autumn, with differences in the time between stations. Ingestion rates 21 on phytoplankton showed a significant trend of increase with chlorophyll-a concentration in the water, 22 with a saturation response at around 240 µg C l-1. Specific ingestion rates ranged between 0.04-2.05 µg C 23 µg-1 nauplii C day-1 and 0.04-3.38 µg C µg-1copepod C day-1. 24 25 Key words: 26 Copepod nauplii, ingestion rates, gut fluorescence, Cantabrian sea 1 1 INTRODUCTION 2 In the present scenario of general concern about climate change, the need to 3 understand the biogeochemical cycles in the oceans and quantify the importance of all 4 the processes that take part in them is becoming more and more urgent. Research 5 programs on this topic are mostly focused on coastal zones due to their accessibility and 6 special characteristics. Coastal zones are generally very sensitive to any external 7 forcing, so climate changes are therefore likely to have the greatest impact and be 8 experienced first in them, whereas spatio-temporal buffering in the oceans may delay 9 evidence of climate change for decades or even centuries (Sündermann et al. 2001). 10 From 1993, a monthly sampling has been conducted in the Central Cantabrian 11 Sea (North of Spain). During this period, several studies relating physical and chemical 12 parameters have been carried out in this area (eg. Llope et al. in press, Stenseth et al. 13 submitted). It has also been evaluated the numerical importance and feeding impact of 14 different taxonomical groups, such as, fish larvae (González-Quirós & Anadón 2001), 15 appendicularians (López-Urrutia et al. 2003), mesozooplanktonic copepods (Huskin et 16 al. 2006), protozoa (Quevedo & Anadón 2000), and bacteria (Serret et al. 1999, 17 González et al. 2003). This means a significant amount of reference data, allowing us to 18 establish an observation strategy and detect future changes in the coastal zone. There is 19 available information about most of the main zooplanktonic groups in the area, being 20 maybe the major deficiency the lack of studies dealing with small copepods and nauplii. 21 Copepods are the most abundant group in the metazooplankton and they have 22 been one of the main focuses of oceanographic studies during last decades. In our study 23 area, mesozooplanktonic copepods abundance and feeding rates on phytoplankton have 24 been estimated during an annual cycle by Huskin et al. (2006), but this study did not 25 include the fraction <200 µm (mostly nauplii and copepodites). In fact, we are aware of 2 1 very few studies of ingestion rates for the naupliar phase (reviewed in López et al. 2 2006). And most of the published data come from studies with cultures of copepods and 3 phytoplankton in laboratory, so results are difficult to extrapolate to natural conditions. 4 Nauplii have received relatively little attention despite the fact that they are more 5 abundant than copepodites and copepods in the field and that their success in the 6 plankton will ultimately determine recruitment into the copepodite phase and, 7 consequently, the population dynamics (Torres & Escribano 2003). To our knowledge, 8 there are no studies in the literature regarding the seasonal changes in nauplii feeding 9 rates. However, seasonal changes in their abundance have been reported for other zones 10 (reviewed in Turner 2004), although data are still rather scarce due to the common use 11 of 200 µm mesh nets to sample metazooplankton. The bias produced by the use of such 12 large pore nets to sample copepods assemblages has been reported several times (e.g. 13 Calbet et al. 2001, Turner 2004), as they seize inefficiently nauplii, copepodites and 14 adult copepods from the smallest species. This lack of information about copepod 15 nauplii (and small copepods) makes it impossible to correctly evaluate the importance 16 of copepods in the oceanic carbon cycles. 17 The scarcity of data about nauplii ingestion rates in natural communities can be 18 explained by the methodological difficulties to work with such small organisms. In a 19 previous work (López et al. 2006), it has been described a series of adaptations for the 20 gut fluorescence method (Mackas & Bohrer 1976) so it can be used with copepod 21 nauplii. In that work, it has been discussed the choice of the gut fluorescence method to 22 measure nauplii ingestion rates on phytoplankton and the advantages and weaknesses of 23 this technique. 24 In this study, it has been used the previous mentioned methodology to measure 25 herbivory ingestion rates of nauplii, copepods and copepodites from the <200 µm 3 1 fraction during an annual cycle. The functional responses of this fraction feeding on 2 phytoplankton were studied, calculated the impact caused on phytoplankton stock and 3 compared with data obtained for larger copepods by Huskin et al. (2006). It has also 4 been studied the seasonal changes in the abundance of the main metazooplankton 5 groups and related to phytoplankton abundance and water temperature. 6 7 MATERIALS AND METHODS 8 The study took place in a transect of 3 stations (E1, E2 and E3) off Cudillero in 9 the southern Bay of Biscay (Figure. 1). This zone exhibits a very dynamic hydrography 10 (described in Llope et al. in press) and the stations show significant differences in spite 11 of their proximity. E1 (65 m depth) is a coastal station influenced by freshwater 12 discharges, tidal currents and frequent wind-driven upwelling during summer. E2 (130 13 m) is located on the continental shelf and as such is also affected by upwelling events 14 although less intensively than E1. E3 (850 m) is on the slope and is the most oceanic as 15 it is only marginally affected by coastal processes except for the occasional appearance 16 of the Iberian Poleward Current and the indirect effect of upwellings, probably by 17 offshore advection (Stenseth et al. submitted). 18 Sampling was done monthly during 2003. At every station, physical and 19 chemical parameters were measured and samples were taken to determine, chlorophyll- 20 a (chl-a) concentration, mesozooplankton biomass, micro and mesozooplankton 21 taxonomy and nauplii and copepodites gut contents. In station E2 samples were taken 22 also for phytoplankton taxonomy and primary production. 23 24 Vertical profiles of temperature and salinity were obtained with a CTD from a depth of 50, 100 and 500m in E1, E2 and E3 respectively. 4 1 Chl-a concentration was determined fluorometrically. Water samples were 2 collected with 5 l Niskin bottles at 6 - 10 different depths from surface to the end of the 3 photic layer. Samples were carried to the laboratory in cold conditions and filtered onto 4 GF-F filters. Filters were frozen and extracted in 5 ml 90% acetone during 24 h in dark 5 and cold conditions. Chl-a concentration was measured with a Turner Designs 10 6 fluorometer following the method of Yentsch & Menzel (1963). 7 Primary production was determined by incubating with 14 C water from 3 8 different depths (surface, chlorophyll maximum and limit of the photic layer). Water 9 samples were inoculated with 370 kBq (10 µCi NaH14CO3) and incubated for 2 h. Three 10 light bottles and one dark bottle (control) were incubated for each depth. Temperature 11 and light for each treatment were simulated following preliminary study of the CTD 12 casts. After incubation, samples were filtered onto GF-F filters, exposed for 12 hours to 13 concentrated HCl fumes to remove inorganic 14 scintillation counter. Quenching was corrected by the internal standard method. There 15 was a problem with primary production experiment during August so there is a gap on 16 data. 14 C, and counted in a Wallac 1409 17 Water samples for phytoplankton species identification were collected at 3 18 different depths (surface, chlorophyll maximum and limit of the photic layer); they were 19 preserved with 2% final concentration Lugol´s iodine solution. Subsamples (100 ml) 20 were settled (Utermöhl method) and counted with an inverted microscope. 21 At every station, one WP2 net (37 cm diameter, 200µm mesh) was deployed to 22 50 (station E1), 100 (station E2) or 200 m (station E3) to sample mesozooplankton for 23 biomass quantification. Cod end contents were kept in 250 ml plastic bottles and carried 24 to the laboratory where they were screened through 200, 500 and 1000 µm meshes to 25 create three different size fractions. Each fraction was filtered onto GF-A pre- 5 1 combusted and pre-weighed filters, maintained for 48 h at 60ºC and weighed. Biomass 2 was expressed as mg dry weight m-3. 3 Two net tows were carried out at every station with a 53µm mesh net to collect 4 zooplankton from the upper 50 m. The first net tow was devoted to metazooplankton 5 taxonomic composition and the second to gut fluorescence analysis. Some net samples 6 from October, November and December were lost so there is some gaps on data. The 7 cod end content from the first tow was screened through 200 and 30 µm meshes and 8 both samples were fixed with 4 % buffered formaldehyde and determined under a 9 stereomicroscope to the level of main taxonomic groups. The cod end content from the 10 second tow was fractionated in the same way and samples from the <200 µm fraction 11 were filtered onto mesh filters, frozen in liquid nitrogen and kept frozen until analysis. 12 Gut fluorescence was measured for nauplii and copepods <200 µm (cop <200 µm, 13 includes copepodites and copepods from the <200 µm, as we did not distinguish 14 between both of them) following Mackas & Bohrer (1976) and the adaptations 15 described in López et al. (2006). For each station, 3 groups of 20 nauplii and 6 groups 16 of 10 cop <200 µm were analysed. The samples were extracted in 120 µl of acetone (90 17 %) for 24 h at 4 ºC and measured with a Turner Designs 700 fluorometer with a 18 minicell adapter kit. 19 Ingestion rates were calculated with the formula: 20 I=kG 21 where k is the gut clearance coefficient and G is gut content (expressed as ng chl-a eq. 22 ind-1). 23 24 25 To calculate k we use the empirical relationship with temperature (T) proposed by Dam & Peterson (1988) for adult copepods: k = 0.0117 + 0.0018 T 6 1 A previous study with copepod nauplii (López et al. 2006) found no differences 2 between gut evacuation rates obtained with nauplii in the laboratory and the rates 3 obtained with the previous equation. 4 Groups of at least 40 nauplii and 40 copepodites from every sample were 5 measured by taking photographs of the sample under the stereomicroscope and using 6 image analysis software. Average dry weight was estimated for each sample using the 7 following relationships: 8 Log dry weight (µg) = 2.1034 log nauplii total length (µm) - 5.2105 9 Log dry weight (µg) = 2.6757 log copepodite prosome length (µm) - 6.7625 10 As relationships found in the literature are usually obtained for only one species 11 (reviewed in Mauchline 1998), we calculated the above mentioned ones with data from 12 the study of Klein Breteler et al. (1982), for four species of copepods very abundant in 13 our study area. Their graphs were scanned, all data from them were obtained with image 14 analysis software and new relationships were obtained by plotting all data together. 15 They were used to calculate dry weight for the mix of copepods found in our samples. 16 They were compared with relationships presented in Mauchline (1998) and it was 17 checked that parameters were in the same range as others from different studies. 18 19 To convert chlorophyll concentration in C units, as an index was not available for each station and date, a value of 50 was used for all samples (Taylor et al. 1997). 20 The equations for the different types of functional responses (Holling 1965) 21 were fitted by the least-squares criterion to the ingestion data. For the type I fit 22 (rectilinear model) we followed the procedure by Rothhaupt (1990) to calculate where 23 the deflection point should be, and then we obtained the fit for the combination of the 24 two linear regressions: 25 I aC , when C Cd 7 I Im ax , when C Cd 1 2 where I is the specific ingestion rate (µg C µg-1 nauplii C d-1), a is a constant, C is the 3 phytoplankton concentration (mg chl-a m-3), Cd is the C at the deflection point and Imax 4 is maximum I, calculated as the I average value for C > Cd. 5 For type II we used the Ivlev (1961) equation: I Im ax1 exp aC Im ax 6 7 where I is the specific ingestion rate, Imax is asymptotic maximum I, a is a constant and 8 C is the phytoplankton concentration. 9 And the logistic equation for type III model: I Im ax 1 expKc C a 10 11 where Imax is asymptotic maximum I, C is the phytoplankton concentration, Kc is a 12 constant defined as the food concentration for I Im ax 2 , and a is a constant. 13 To compare between models, minimization of the mean-square error (MSE) was 14 used as the criterion for goodness of fit. The significance of differences in variances 15 among regressions was tested by a two-tailed F-test on the MSE (Rothhaupt 1990, 16 Mullin et al. 1975). 17 18 RESULTS 19 Vertical profiles of temperature and chl-a concentration are shown in figures 2 20 and 3 respectively. The hydrographic features of the study area are those of a typical 21 temperate sea, being the main characteristic the transition from the winter-spring mixing 22 to the summer-autumn stratification with the development of a thermocline at about 40 23 m. A more detailed description about physical and chemical characteristics is presented 24 in Llope et al. (). As a special feature, it has been observed during February in E3, the 25 appearance of a low salinity water mass in the upper 50 m of the water column (salinity 8 1 profile not shown). In this water, a winter bloom developed reaching the highest 2 chlorophyll concentration for the whole annual cycle. 3 Metazooplankton and phytoplankton abundance 4 Copepods have been the most numerous group of the metazooplankton in both 5 fractions (Table 1 and 2). They represented on average the 72,5 % of the total 6 abundance in the >200 µm fraction and the 93 % including both fractions. The 81 % of 7 the total copepods belonged to the <200 µm fraction. Only cirripeds larvae have 8 outnumbered them in the fraction >200 µm during April in E1 and February in E2. 9 Appendicularians have also presented really high abundances during most of the year 10 and doliolids during late summer and autumn, reaching the highest value during 11 October in E3. 12 As copepods represented the majority of the mesozooplankton, we can interpret 13 changes in the relative biomass of each fraction (Figure 4) as changes in copepod 14 community size structure. It is necessary, however, taking into account the cases in 15 which cirriped larvae have been rather abundant (February in the three stations and 16 April in E1). Cirriped larvae would be mostly comprised in the 200 - 500 µm fraction. 17 There was a significant increase in the biomass of this fraction during February in E2 18 when it was found the highest cirriped larvae abundance. The other more abundant 19 groups were those belonging to the gelatinous zooplankton, that with their high water 20 content were not expected to account for a significant amount of sample dry weight. 21 Observed changes in copepods number were not directly related with changes in 22 biomass. The highest mesozooplankton biomass was found in spring, when a shift in the 23 community size structure was observed, increasing the abundance of large sized 24 copepods, and resulting in a lower total number than that of periods when small species 25 dominate. Another point is the different kind of sampling used for both parameters. Net 9 1 tows have been deployed to 50 m in the three stations for taxonomic analysis and gut 2 contents, while they have been deployed to 50, 100 and 200 m in E1, E2 and E3 for 3 mesozooplankton biomass. So in E2 and E3 a non-homogeneous distribution of 4 copepods in the water column would lead to non comparable results with both methods. 5 This can be the reason why in February in E3 we have found the highest number of 6 copepods for the whole cycle and it does not match with the data obtained for biomass. 7 Seasonal changes in the abundance of the whole copepod community (Figure 5), 8 were mainly due to changes in nauplii numbers. There are different patterns for the 9 three stations. In the coastal one it was observed a significant increase in the number 10 during June, while it did not occur until August in E2 and September in E3. Afterwards, 11 the number remained high until December in the three stations. 12 It was analysed the relationship between nauplii abundance and: (1) temperature 13 and (2) chlorophyll concentration in the water, by linear regression. The objective of 14 these analyses was to identify the variables that are driving copepods population 15 dynamics. The increase in nauplii abundance during favourable conditions is also 16 dependant on the number of adult copepods; so, apart from the total nauplii abundance, 17 we have also performed the analyses with the relationship “number of nauplii/ number 18 of copepods” (nau/cop) for each period. It was found a significant effect for temperature 19 in total nauplii (r2 = 0.277, p = 0.001) and nau/cop (r2 = 0.126, p = 0.039), which 20 affected positively their number. Chlorophyll concentration in the water only showed a 21 significant relationship with total nauplii (r2 = 0.224, p = 0.005), showing a decrease in 22 the density of nauplii at high chlorophyll concentrations. 23 The highest phytoplankton abundance in E2 was reached during spring bloom 24 (Figure 6), when diatoms were dominant in the community. A second peak in the 25 abundance was reached in late summer (August and September), when the most 10 1 numerous groups were diatoms and crysophyceae. It was possible to compare the 2 pattern of integrated chl-a concentration with that of abundance of phytoplankton cells 3 in E2, as both data were available. Both patterns did not coincide; while the summer 4 increase in phytoplankton started in August for abundance, it was not until September 5 for chl-a concentration. 6 Copepod abundance followed a different pattern than chlorophyll concentration 7 and phytoplankton abundance in the three stations. However, there was a 8 correspondence in E2 between August increase in number of phytoplankton cells and 9 increase in number of copepods. 10 Grazing rates and functional responses 11 Nauplii and cop <200 µm gut contents ranged between 0.004 - 0.082 ng chl-a 12 eq. ind-1 and 0.003 - 0.315 respectively. Carbon ingestion rates of nauplii and cop <200 13 µm (Figures 7 and 8 respectively) translate into a grazing of 0.3 - 9.6 % of 14 phytoplankton stock daily and 0.49 – 19.9 % of primary production in E2 (Figure 9). 15 Equations for the three models of functional responses were fitted to ingestion 16 data (Figure 10), and parameters were obtained by the least-squares criterion (Table 3). 17 Although the type III model showed the lower MSE, all of them were quite similar and 18 we did not find significant differences in explained variance between models (p > 0.3 in 19 all cases). 20 Both, nauplii and cop <200µm, showed a saturation response at around 2 - 4 mg 21 chl-a m-3. Calculated Imax could be significantly lower than the real one due to the 22 shortage of data in high chlorophyll concentrations. This problem is more evident in the 23 case of cop <200 µm; attending to Figure 10 we could expect an Imax of at least 2 µg C 24 µg-1 copepod C day-1. 25 11 1 2 DISCUSSION 3 Copepods abundance and seasonal changes in the community 4 During all the year, nauplii dominated copepods community in terms of 5 abundance (67 %). Similar results have been found by other authors in different areas; 6 nauplii accounted for 65% of the total copepods community in a coastal area of the NW 7 Mediterranean Sea (Calbet et al. 2001) and 63% in coastal waters off Jamaica (Hopcroft 8 et al. 1998). Nauplii densities during the annual cycle (on average 12 nauplii l-1) are in 9 the same range than others in coastal zones (Roff et al. 1995, Calbet et al. 2001, 10 Pedersen et al. 2005), but sometimes more than an order of magnitude lower than more 11 productive systems ones (Lucic et al. 2003, Uye et al. 1996). The high abundance of the 12 juvenile stages stresses the importance of including the fraction of copepods not 13 retained by the 200 µm mesh in the community studies. However, it is possible that 14 when using 53 µm mesh nets, nauplii are still being under sampled. Lucic et al. (2003), 15 studied nauplii abundance in the Adriatic Sea by sampling with Niskin bottles and 16 found that those belonging to the group with body length <80 µm could account for 17 30% of total nauplii. In our samples, such small nauplii are rather scarce, even though, 18 small sized copepod species such as Oithona nana and Oncaea media are abundant in 19 the study area. Thus, we suspect that nauplii from these species are not being kept 20 efficiently in the net. Lucic et al. (2003) working on the principle that they had found a 21 significant correlation between small nauplii and bacteria abundances, suggested that 22 they had a mainly bacterivorous diet, but they did not find this correlation with larger 23 nauplii. Although bacterivory had been previously described by Turner & Tester (1992) 24 and Roff et al. (1995), it is still not clear under what circumstances this occurs, as 25 Sommer et al. (2000) found that particles <2 µm escaped predation by copepod nauplii. 12 1 If smallest nauplii are actually feeding on bacteria, their under sampling would not 2 change the estimated impact on phytoplankton community. But, even in the case that 3 they were feeding on phytoplankton, we would not expect the real community ingestion 4 rate to be significantly higher, due to the smaller size of this possibly under sampled 5 fraction and consequently lower gut contents. 6 It would be logical to expect the number of copepods influenced by availability 7 of preys. In spite of this, we have found a negative relationship between nauplii 8 abundance and chlorophyll concentration in the water. It could be explained if 9 heterotrophic preys were more abundant when chlorophyll bearing preys decrease. We 10 have not measured the abundance of other potential preys, apart from phytoplankton, 11 but this option does not appear as probable. Other authors have suggested predation 12 (Calbet et al. 2001, Lawrence et al. 2004) and improved competitive advantage of 13 protozoans against metazoans at higher food concentrations (Uye et al. 1996) as causes 14 for the lack of relationship between copepods and nauplii abundances and chlorophyll 15 concentration in the water. Saiz et al. (1999) found an increase in copepods egg 16 production across the natural nutrient gradient from “oligotrophic” oceanic waters to 17 “eutrophic” shelf waters, which did not translate into an increase in the abundance of 18 copepods, and suggested that predation or advection may uncouple production from 19 abundance. Micheli (1999) evaluated bottom-up and top-down controls on consumers. 20 Their results suggest that these controls attenuate through marine food webs, and in 21 general, there may be a weak coupling between phytoplankton and herbivores. 22 On the other hand, the explanation that seems more accurate to us in sight of 23 phytoplankton and zooplankton annual cycles is that a combination of three factors 24 drives copepod population dynamics: (1) water temperature, (2) quantity and (3) quality 25 of available food. Some observations have shown that environmental temperature rather 13 1 than phytoplankton abundance controls egg production in copepods, so daily rates of 2 egg production increase with temperature to a maximum but then decrease with further 3 increases in temperature (Mauchline 1998). We have found a positive influence of 4 temperature in nauplii abundance and this latter could be used as an indicator of egg 5 production. Other studies have pointed out that quality as well as quantity of food 6 available is important; high quality encouraging production of successive egg masses 7 and clutches (see references in Mauchline 1998). 8 A recent study from Ianora et al. (2004) has found that dominant diatom species 9 impair the reproductive success of their grazers. Identifying the aldehydes that arrested 10 larval development in copepods, they have introduced a new light in the debate about 11 the positive or negative effect of diatoms in copepods populations. Although not all 12 bloom-forming species produce aldehydes, their findings provide a plausible 13 mechanism for the apparent poor timing between spring bloom development and the 14 arrival of the bulk of the copepod stock. When looking for a relationship between 15 nauplii abundance and chl-a concentration in the water (as an indicator of “quantity” of 16 available food) the interference of the “quality” factor would explain the impossibility 17 of finding a significant relationship. 18 These three factors acting together could shift the period of time most suitable 19 for breeding, from spring, with low water temperature and low food quality 20 (phytoplankton assemblages mainly composed by diatoms), although the highest chl-a 21 concentration, to the end of summer, with a higher water temperature and a second 22 increase in chlorophyll caused by growth of higher quality phytoplankton species. In 23 August, when the copepods abundance started to increase, diatoms were again abundant 24 in the phytoplankton, being their populations probably enhanced by the short-lived 25 upwellings that are a common event in the area during summer (Llope et al. in press). 14 1 But their relative abundance were not so high as in spring, and it has been pointed out 2 that a mixed diet serves to dilute the toxin causing lower effects on copepods 3 recruitment (Ianora et al. 2004). Another point is that timescale has been suggested as 4 very important in the negative effects of diatoms (Irigoien et al. 2002), and the shorter 5 period in which they were dominant during summer could not have been enough to 6 cause the same deleterious effects as during spring bloom. 7 As phytoplankton taxonomy is not available for E1 and E3, only chl-a 8 concentration can be used to compare copepods and phytoplankton annual cycles. 9 Although it has been observed for E2 that chl-a is sometimes an imperfect index of the 10 availability of phytoplankton, it gives us a guiding idea and we would expect that 11 succession follows a quite similar pattern than in E2. The copepod seasonal distribution 12 in E1 and E3 matches with the before explained theory. In E1 where chl-a concentration 13 remains high during all the year the breeding seasonality would be mainly controlled by 14 temperature and food quality for the majority of copepod species, while in E3 the lower 15 phytoplankton concentration during the beginning of summer would delay it until the 16 end of summer when chl-a increases and water temperature is still high. 17 During February in E3 it has been observed a water mass with special 18 characteristics. In the upper 50 m, water had lower salinity and higher chlorophyll 19 concentration than expected, which not correlated with data for E2. It had been 20 previously described the occurrence of slope fronts in the area (González-Quiros 1999, 21 González 2003). The presence of this kind of structure in outer waters could act as a 22 barrier for the surface transport of fresh water from Nalón River, justifying the 23 accumulation of this “low-salinity water” just before the front. During the previous days 24 to the sampling, the meteorological conditions in the area had been characterised by 25 weak winds (Llope, personal communication), which would have favoured the 15 1 formation and maintenance of this structure. Differences found during this month 2 between copepod abundance and biomass were possibly due to the majority of 3 copepods of the water column accumulated in the upper 50 m where the bloom 4 developed. Nutrients analysis (data not shown) indicated that the bloom was in an 5 advanced development stage. 6 Grazing rates 7 Ingestion rates obtained in this study should be considered as approximations as 8 the method used have some potential source of errors, such as calculation of gut 9 evacuation rate, and the possibility of copepods presenting diel feeding periodicities 10 which have not being assessed in this study. Also, values obtained with this method are 11 usually considered as minimum estimates due to the uncertainty about pigment 12 degradation in copepod guts (discussed in López et al. 2006). Although it is still not 13 clear under what circumstances and to what extend chlorophyll a is degraded to 14 colourless products, some authors apply an average value of 33 % destruction to correct 15 their estimates (Dam & Peterson 1988). Even though, gut fluorescence method provides 16 valuable results and allows measuring ingestion rates of small nauplii in the field. 17 When preparing samples to analyze gut fluorescence, nauplii were picked 18 without paying attention to development stage. Sorting only nauplii from feeding stages 19 would have been rather difficult and time consuming. Also, there is not a consensus 20 about which is the first nauplius stage to feed and it seems that, although most of 21 calanoid copepods start to feed during NIII, there are differences between species 22 (Mauchline 1998), cyclopoid and poecilostomatoid nauplii in contrast, could start to 23 feed immediately after hatching (Uchima & Hirano 1986, Paffenhöfer 1993). Thus, 24 when picking and counting all kinds of nauplii we were ensuring to obtain the real 25 community ingestion rate on phytoplankton, although individual rates would be 16 1 underestimated. Relationships between copepods and nauplii numbers give us an idea of 2 the high mortality rates during development (e.g. due to high predation on nauplii as 3 described in Liang & Uye, 1996). This implies that the first naupliar stages should be 4 much more abundant than the last ones, translating into a significant underestimation of 5 the individual ingestion rates. 6 Specific ingestion rates ranged between 0.03-1.71 µg C µg-1 nauplii C day-1 and 7 0.03-2.82 µg C µg-1copepod C day-1. As we could not find in the literature field studies 8 regarding nauplii grazing rates in temperate seas, we compare our data with the scarce 9 information available about this issue, coming from studies carried out in high latitudes 10 and mostly focused in the largest fraction of nauplii. Our data were in the same range 11 than values presented in those studies: 0.11 - 0.46 (Irigoien et al. 2003), 0 - 0.32 12 (Hansen et al. 2000), 0.28 - 0.52 (Tackx et al. 1990), 0.79 - 2.8 (White & Roman, 1992) 13 and 0.08 - 0.29 µg C µg-1 nauplii C day-1 (Uitto 1996). Ingestion rates found for cop 14 <200 µm are also in the same range, although sometimes higher, than those reported in 15 the literature. Mauchline (1998) made a review of copepod ingestion rates on 16 phytoplankton and reported mean values ranging between 0.02 - 1.83 µg C µg-1copepod 17 C day-1 for copepodites and 0.013 - 1.5 for adult copepods. 18 Functional responses 19 The study of functional responses provides a useful tool to predict the trophic 20 impact caused by copepods when specific experiments are not possible to conduct. Two 21 conceptual models (Lam & Frost 1976; Lehman 1976) pointed out that a type III 22 functional response (Holling 1965) is the one that maximizes the net gain of energy for 23 a copepod. They predict that below a critical food concentration the energy expenditure 24 of the feeding process is higher than the gain from the assimilation of the food 25 collected. Thus, an animal in this situation may reduce the feeding activity to minimize 17 1 the energy loss or even cease it. We have found that a type III model was the one that 2 best fit our data. However, differences between models were not significant, and in 3 Figure 10 we can observe type III plot have a positive “Y intercept”. Thus, it would 4 predict an unreal situation, individuals with chlorophyll in their guts when there is no 5 phytoplankton in the environment. Although López et al. (2006) observed a better fit to 6 a type III model with nauplii feeding on phytoplankton cultures; we think in this case 7 type II would be more suitable. The difference between type II and type III models is a 8 reduction in the ingestion at low food concentrations predicted by type III. We have 9 obtained the functional responses by studying only the ingestion on autotrophic preys, 10 but most copepods feed as omnivores (Turner 2004), and the abundance of 11 heterotrophic preys could explain the high data variability around the values predicted 12 by the model. During each sampling period, heterotrophic preys could be completing 13 copepods diet up to a different level. On the other hand, the presence of different 14 choices when phytoplankton abundance is low, would explain that we do not observe a 15 type III response, as copepods could continue filtering at the same rate, taking 16 advantage of all kinds of preys and not compromising their energy balance. 17 Experiments involving all kinds of preys would be necessary to elucidate this aspect. 18 Both stages showed saturation responses at around 240 µg C l-1, although the 19 scarcity of samples at higher concentrations could have biased the calculation. Such 20 high concentrations can only be found in our study area during the beginning of the 21 spring phytoplankton bloom. Frost (1972) found a similar saturation concentration for 22 adult females. In contrast, experiments with nauplii in laboratory (López el al. 2006) 23 showed 2 to 3 times higher saturation concentrations. López et al. had already 24 suggested, that nauplii in natural conditions, where preys with more suitable sizes are 18 1 available, would probably reach saturation responses at lower chlorophyll 2 concentrations. 3 Nauplii vs. copepods 4 The study of Huskin et al. (2006) carried out during 1998 (same methodology 5 and area) for copepods >200 µm (cop >200 µm), gave us a valuable opportunity to 6 compare ingestion rates for the different sizes and development stages. Huskin et al. 7 found that cop >200 µm in stations E1 and E2 ingested on average 7 % of chlorophyll 8 standing stock daily, ranging between 0.36 and 25.5 %, and between 1 and 53 % for 9 daily primary production in E2. The ingestion rates we have found for the <200 µm 10 fraction averaged 2.8 % of chlorophyll standing stock and 5.7 % of daily primary 11 production. Thus, the total copepods ingestion on phytoplankton would never reach 12 100% of primary production, although during a few months in the year it would surpass 13 50%, having then a significant role in the control of phytoplankton populations. Not 14 including the fraction <200 µm when measuring copepods community ingestion rates, 15 would imply an underestimation of about 30 %. A similar proportion of C ingested by 16 the different stages of copepods was obtained by Sommer et al. (2000), as they found 17 that copepods ingestion on seston particles was four times higher than that of nauplii. 18 To compare individual ingestion rates between the different sizes and stages of 19 copepods, we have transformed ingestion rates reported by Huskin et al. to specific 20 ingestion rates. As copepods length and/or weight had not been measured in the 21 previous study, a sample was taken in station E2 with a WP-2 net (200 µm mesh) and 22 fractionated in the same way that they did (200-500 µm, 500-1000 µm and > 1000 µm). 23 In the laboratory, a sample of 60 copepods from each fraction was measured under a 24 stereomicroscope, obtaining an average length for each fraction and calculating C 25 weight with the same equation used for cop <200 µm. All the data from both studies 19 1 have been plotted in Figure 12. The data for cop >200 µm are only guiding values as we 2 made an error by using the same average size for all the samples. Previous studies have 3 suggested nauplii may have weight-specific ingestion rates 3 - 4 times higher than 4 adults (Lonsdale et al. 1996, Paffenhöfer 1971). We have calculated average specific 5 ingestion rates of 0.56 for nauplii, 0.71 for cop <200 µm, 0.42 for cop 200-500 µm, 0.29 6 for cop 500-1000 µm and 0.09 for cop >1000 µm. There is a trend of decreasing 7 specific ingestion rate with increasing body weight. The presence of non-feeding stages 8 in the nauplii assemblage would have lead to an underestimation of individual ingestion 9 rates that would explain the lower differences found between large copepods and nauplii 10 rates in comparison with previous studies, as to perform grazing experiments in the 11 laboratory only feeding stages are selected. 12 13 This study has allowed a deeper insight in copepods population dynamics in the 14 Cantabrian sea, by including the fraction < 200 µm for the first time. The study of the 15 whole community has been necessary for a better understanding of population 16 dynamics. However, when modelling carbon fluxes in the ocean, it is necessary to have 17 in mind that all stages of copepods must not be considered as an only group but as 18 different compartments. Due to the small size of their pellets, nauplii are not likely to be 19 important participants in the biological pump as adult copepods, but they may play an 20 important role in surface recycling processes (Green et al. 1992). They have also been 21 suggested to be critical intermediaries between “classical” and microbial food webs as 22 they can feed on pico and nanoplankton (Turner & Tester 1992, Roff et al. 1995) that 23 larger copepods may be unable to consume directly. We have found another important 24 zooplanktonic component in the area were appendicularians. Their grazing rates have 25 been estimated in a previous seasonal study (López-Urrutia et al. 2003), finding they 20 1 translated into, on average, 8 % of the daily primary production, reaching values as high 2 as 60 %. This means gelatinous zooplankton could be ingesting in some cases a higher 3 amount of phytoplankton than copepods. They are suggested to exploit the same size 4 fraction as nauplii, thus, they would also act as a nexus between both “classical” and 5 microbial food webs. In contrast, they produce larger fecal pellets which would not 6 participate in recycling processes. 7 Although the sparse information available on copepod nauplii makes it difficult 8 to assess their quantitative and ecological importance, this study has provided a first 9 approach of their impact on phytoplankton populations in temperate seas, which will 10 help us to reach an unbiased global view of the role of metazooplankton as consumers 11 of phytoplankton. 12 13 ACKNOWLEDGEMENTS 14 This work was funded by the collaboration specific agreement between 15 Universidad de Oviedo and Instituto Español de Oceanografia: “Control a largo plazo 16 de las condiciones químico-biológicas en la plataforma continental de Asturias” (SV97 17 IEO1), the CICYT project MAR1999-1072-C03-03, the FICYT project “Apoyo a 18 Grupos de Excelencia” and a FPU grant by MEC to E.L. We wish to thank the crew of 19 the R/V “Jose Rioja” for their help to collect samples. Thanks are also given to the 20 Group of Biological Oceanography of the Universidad de Oviedo for their help with the 21 sampling and I. Huskin and D. Fernández for their technical advice during the first 22 stages of this study. We are also grateful to J. L. Acuña for his useful comments on an 23 earlier version of the manuscript. 24 21 BIBLIOGRAPHY Baars MA, Oosterhuis SS (1984) Diurnal feeding rhythms in the North Sea copepods measured by gut fluorescence, digestive enzyme activity and grazing on labelled food. Neth J Sea Res 18:97-119 Calbet A, Garrido S, Saiz E, Alcaraz M, Duarte CM (2001) Annual zooplankton succession in coastal NW Mediterranean waters: the importance of the smaller size fractions. J Plankton Res 23:319-331 Christoffersen K, Jespersen AM (1986) Gut evacuation rates and ingestion rates of Eudiaptomus graciloides measured by means of the gut fluorescence method. J Plankton Res 8:973-983 Crawley MJ (1975) The numerical response of insect predators to changes in prey density. J Anim Ecol 44:877-892 Cushing DH (1978) Upper trophic levels in upwelling areas. In: Boje R and Tomczak M (eds) Upwelling ecosystems. Springer, New York, p 101-110 Dam HG, Peterson WT (1988) The effect of temperature on the gut clearance rate constant of planktonic copepods. J Exp Mar Biol Ecol 123:1-14 Frost BW (1972) Effects of size and concentration of food particles on the feeding behavior of the marine planktonic copepod Calanus pacificus. Limnol Oceanogr 17:805-815 González N, Anadón R, Viesca L (2003) Carbon flux through the microbial community in a temperate sea during summer: role of bacterial metabolism. Aquat Microb Ecol 33:117-126 González-Quirós R (1999) Distribución del ictioplancton en el Cantabrico central y ecología alimentaria de larvas de Micromesistius poutassou (Risso, 1826). PhD thesis, Universidad de Oviedo, Oviedo, Spain González-Quirós R, Anadón R (2001) Diet breadth variability in larval blue whiting as a response to plankton size structure. J Fish Biol 59:1111-1125 Green EP, Harris RP, Duncan A (1992) The production and ingestion of faecal pellets by nauplii of marine calanoid copepods. J Plankton Res 14:1631-1643 Hansen BW, Hygum BH, Brozek M, Jensen F, Rey C (2000) Food web interactions in a Calanus finmarchicus dominated pelagic ecosystem- a mesocosm study. J Plankton Res 22:569-588 Holling CS (1965) The functional response of predators to prey density and its role in mimicry and population regulation. Mem Entomol Soc Can 45:3-60 Hopcroft RR, Roff JC, Lombard D (1998) Production of tropical copepods in Kingston Harbour, Jamaica: the importance of small species. Mar Biol 130:593-604 22 Huskin I, López E, Anadón R (2006) Seasonal variation of mesozooplankton distribution and copepod grazing in the Central Cantabrian Sea (Southern Bay of Biscay). Sci Mar 70S1:119-130 Ianora A, Miralto A, Poulet SA, Carotenuto Y, Buttino I, Romano G, Casotti R, Pohnert G, Wichard T, Colucci-D’Amato L, Terrazzano G, Smetacek V (2004) Aldehyde suppression of copepod recruitment in blooms of a ubiquitous planktonic diatom. Nature 429:403-407 Ivlev VS (1961) Experimental ecology of the feeding of fishes. Yale University Press, New Haven, Connecticut, USA Irigoien X, Harris RP, Verheye HM, Joly P, Runge J, Starr M, Pond D, Campbell R, Shreeve R, Ward P, Smith AN, Dam HG, Peterson W, Tirelli V, Koski M, Smith T, Harbour D, Davidson R (2002) Copepod hatching success in marine ecosystems with high diatom concentrations. Nature 419:387-389 Irigoien X, Titelman J, Harris RP, Harbour D, Castellani C (2003) Feeding behaviour of Calanus finmarchicus nauplii in the Irminger Sea. Mar Ecol Prog Ser 262: 193-200. Ivlev VS (1961) Experimental ecology of the feeding of fishes. Yale University Press, New Haven, Connecticut, USA Klein Breteler WCM, Fransz HG, Gonzalez SR (1982) Growth and development of four calanoid copepod species under experimental and natural conditions. Neth J Sea Res 16:195-207 Lam RK, Frost BW (1976) Model of copepod filtering response to changes in size and concentration of food. Limnol Oceanogr 21:490-500 Lawrence D, Valiela I, Tomasky G (2004) Estuarine calanoid copepod abundance in relation to season, salinity, and land-derived nitrogen loading, Waquoit Bay, MA. Est Coast Shelf Sci 61:547-557 Lehman JT (1976) The filter-feeder as an optimal forager, and the predicted shapes of feeding curves. Limnol Oceanogr 21:501-516 Liang D, Uye S (1996) Population dynamics and production of the planktonic copepods in a eutrophic inlet of the Inland Sea of Japan. III. Paracalanus sp. Mar Biol 127:219-227 Llope M, Anadón R, Viesca L, Quevedo M, González-Quirós R, Stenseth N (in press) Hydrography of the southern Bay of Biscay shelf-break region: integrating the multi-scale physical variability over the period 1993-2003. J Geophys Res B López E, Anadón R, Harris RP (2006) Functional responses of copepod nauplii using a high efficiency gut fluorescence technique. Mar Biol DOI: 10.1007/s00227-0060387-0 23 López-Urrutia A, Irigoien X, Acuña JL, Harris RP (2003) In situ feeding physiology and grazing impact of the appendicularian community in temperate waters. Mar Ecol Prog Ser 252:125-141 Lucic D, Njire J, Morovic M, Precali R, Fucks D, Bolotin J (2003) Microzooplankton in the open waters of the northern Adriatic Sea from 1990 to 1993: the importance of copepod nauplii densities. Helgoland Marine Research 57:73-81 Mackas D, Bohrer R (1976) Fluorescence analysis of zooplankton gut contents and an investigation of diel feeding patterns. J Exp Mar Biol Ecol 25:77-85 Mauchline J (1998) The biology of calanoid copepods. In: Blaxter JHS, Southward AJ, Tyler PA (eds) Adv Mar Biol. Academic Press, London Micheli F (1999) Eutrophication, fisheries, and consumer-resource dynamics in marine pelagic ecosystems. Science 285:1396-1398 Mullin MM, Fuglister-Steward E, Fuglister FJ (1975) Ingestion by planktonic grazers as a function of concentration of food. Limnol Oceanogr 20:259-262 Paffenhöfer GA (1993) On the ecology of marine cyclopoid copepods (Crustacea, Copepoda). J Plankton Res 15:185-211 Paffenhöfer GA (1971) Grazing and ingestion rates of nauplii, copepodites and adults of the marine planktonic copepod Calanus helgolandicus. Mar Biol 11:286-298 Pedersen SA, Ribergaard MH, Simonsen CS (2005) Micro- and mesozooplankton in Southwest Greenland waters in relation to environmental factors. J Mar Syst 56:85-112 Quevedo M, Anadón R (2001) Spring microzooplankton composition, biomass and potential grazing in the Central Cantabrian coast (Southern Bay of Biscay). Oceanologica Acta. 23:297-310 Roff JC, Turner JT, Webber MK, Hopcroft RR (1995) Bacterivory by tropical copepod nauplii: extent and possible significance. Aquat Microb Ecol 9:165-175 Rothhaupt KO (1990) Changes of the functional responses of the rotifers Brachionus rubens and Brachionus calyciflorus with particle sizes. Limnol Oceanogr 35:2432 Saiz E, Calbet A, Irigoien X, Alcaraz M (1999) Copepod egg production in the western Mediterranean: response to food availability in oligotrophic environments. Mar Ecol Prog Ser 187:179-189 Serret P, Fernández E, Sostres JA, Anadón R (1999) Seasonal compensation of microbial production and respiration in a temperate sea. Mar Ecol Prog Ser 187:43-57 Sommer F, Stibor H, Sommer U, Velimirov B (2000) Grazing by mesozooplankton from Kiel Bight, Baltic Sea, on different sized algae and natural seston size fractions. Mar Ecol Prog Ser 199:43-53 24 Stenseth NC, Llope M, Anadón R, Cianelli L, Chan K-S (submitted) Seasonal plankton dynamics along a cross-shelf gradient. Sündermann J, Beddig S, Huthnance J, Mooers CNK (2001) Impact of climate change on the coastal zone: discussion and conclusions. Clim Res 18:1-3 Tackx MLM, Bakker C, van Rijswijk P (1990) Zooplankton grazing pressure in the Osterschelde (The Netherlands). Neth J Sea Res 25:405-415 Taylor AH, Geider RJ, Gilbert FJH (1997). Seasonal and latitudinal dependencies of phytoplankton carbon to chlorophyll ratios: results of a modelling study. Mar Ecol Prog Ser 152, 51-66 Torres CG, Escribano R (2003) Growth and development of Calanus chilensis nauplii reared under laboratory conditions: testing the effects of temperature and food resources. J Exp Mar Biol Ecol 294:81-99 Turner JT, Tester PA (1992) Zooplankton feeding ecology: bacterivory by metazoan microzooplankton. J Exp Mar Biol Ecol 160:149-167 Turner JT (2004) The importance of small planktonic copepods and their roles in pelagic marine food webs. Zool Stud 43:255-266 Uchima M, Hirano R (1986) Food of Oithona davisae (Copepoda: Cyclopoida) and effect of food concentration at first feeding on the larval growth. Bull Plankton Soc Japan 33:21-28 Uye S-I, Nagano N, Tamaki H (1996) Geographical and seasonal variations in abundance, biomass and estimated production rates of microzooplankton in the Inland Sea of Japan. J Oceanogr 52:689-703 Uitto A (1996) Summertime herbivory of coastal mesozooplankton and metazoan microplankton in the northern Baltic. Mar Ecol Prog Ser 132:47-56 White JR, Roman MR (1992) Seasonal study of grazing by metazoan zooplankton in the mesohaline Chesapeake Bay. Mar Ecol Prog Ser 86:251-261 Yentsch CS, Menzel DW (1963) A method for the determination of phytoplankton chlorophyll and phaeophytin by fluorescence. Deep-Sea Res 10:221-231 25 Figure Legends: Figure 1: Location of sampling stations. Figure 2: Vertical profiles of temperature in stations E1, E2 and E3 during JanuaryDecember 2003. Figure 3: Vertical profiles of chl-a concentration (µg chl-a l-1) in stations E1, E2 and E3 during January-December 2003. Figure 4: Seasonal variation in mesozooplankton biomass and copepod abundance in the three sampling stations. Figure 5: Seasonal variation in nauplii, cop <200 µm and cop >200 µm abundance and integrated chl-a concentration in the water column. Figure 6: Seasonal variation in phytoplankton abundance in E2. It was used the term “other groups” for cells belonging to minority groups and those with such a small size that were not possible to identify with the inverted microscope. Figure 7: Nauplii ingestion rates and average chl-a concentration in the water column during the annual cycle. Figure 8: Cop <200 µm ingestion rates and average chl-a concentration in the water column during the annual cycle. Figure 9: Nauplii and cop <200 µm grazing impact on phytoplankton biomass (in E1, E2 and E3) and primary production (in E2) during the annual cycle. Figure 10: Nauplii and cop <200 µm specific ingestion rates as a function of chl-a concentration in the water. Equations for functional responses are fitted to data. Type 1 is denoted by short dashed line; type 2 by long dashed line; and type 3 by solid line. Figure 11: Specific ingestion rates for all the stages and size fractions of copepods in the study area during the annual cycle. Data from Huskin et al. (in press) and this study. Average values and sample standard deviations are plotted for each size class. 26 Figure 1 27 DEPTH (m) E1 -25 -50 50 100 150 200 250 300 350 DEPTH (m) E2 -50 -100 50 100 150 200 250 300 350 E3 DEPTH (m) -50 -100 -150 50 100 150 200 250 300 350 JULIAN DAYS Figure 2 28 0 DEPTH (m) E1 -50 50 100 150 200 250 300 350 DEPTH (m) 0 E2 -50 -100 50 100 150 200 250 300 350 DEPTH (m) 0 E3 -50 -100 50 100 150 200 250 300 350 JULIAN DAYS Figure 3 29 biomass 200-500 µm biomass 500-1000 µm biomass >1000 µm copepod abundance E1 12000 60 8000 40 4000 20 Ju l Au g Se p Oc t No v De c 0 Ja n Fe b M ar Ap r M ay Ju n 0 40 E2 6000 30 mg m-3 4000 20 2000 copepods m-3 mg m-3 80 16000 copepods m-3 100 10 Ju l Au g Se p Oc t No v De c 0 E3 10000 8000 15 6000 10 4000 5 2000 0 0 Ju l Au g Se p Oc t No v De c 20 Ja n Fe b M ar Ap r M ay Ju n mg m-3 25 copepods m-3 Ja n Fe b M ar Ap r M ay Ju n 0 Months Figure 4 30 nauplii copepods <200 µm copepods >200 µm mg chl-a m-2 2500000 E1 160 120 1500000 80 1000000 mg chl-a m-2 individuals m-2 2000000 40 500000 0 Ju l Au g Se p Oc t No v De c Ja n Fe b M ar Ap r M ay Ju n 0 3000000 E2 120 2000000 80 60 1000000 40 mg chl-a m-2 individuals m-2 100 20 Ju l Au g Se p Oc t No v De c 0 Ja n Fe b M ar Ap r M ay Ju n 0 4000000 E3 200 2000000 100 1000000 0 Ju l Au g Se p Oc t No v De c 0 mg chl-a m-2 3000000 Ja n Fe b M ar Ap r M ay Ju n individuals m-2 300 Months Figure 5 31 other groups cryptophyceae prymnesiophyceae dictiochophyceae crysophyceae ciliates diatoms dinophyceae 2000000 1000000 c De v No t Oc p Se g Au l Ju n Ju ay M r Ap ar M b Fe n 0 Ja number of cells cm-2 3000000 Months Figure 6 32 E1 0.2 8 6 0.15 4 0.1 mg cl-a eq m-3 µg C nauplius-1 day-1 0.25 2 0.05 0 0 Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec E2 0.2 8 6 0.15 4 0.1 mg cl-a eq m-3 µg C nauplius-1 day-1 0.25 2 0.05 0 0 Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec E3 0.2 8 6 0.15 4 0.1 mg cl-a eq m-3 µg C nauplius-1 day-1 0.25 2 0.05 0 0 Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec Months Figure 7 33 E1 8 0.6 6 0.4 4 0.2 2 0 0 mg cl-a eq m-3 µg C copepod -1 day-1 0.8 Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec E2 8 0.6 6 0.4 4 0.2 2 0 0 mg cl-a eq m-3 µg C copepod -1 day-1 0.8 Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec E3 8 0.6 6 0.4 4 0.2 2 0 0 mg cl-a eq m-3 µg C copepod -1 day-1 0.8 Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec Months Figure 8 34 % chl-a stock ingested daily nauplii copepods < 200µm 10 8 E1 6 4 2 0 % chl-a stock ingested daily Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec 8 6 E2 4 2 0 % chl-a stock ingested daily Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec 10 8 E3 6 4 2 0 Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec % pp ingested daily 25 20 E2 15 10 5 0 Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec Months Figure 9 35 Specific ingestion (µg C µg-1 copepod C day-1) Specific ingestion (µg C µg-1 nauplius C day-1) 2 1.6 1.2 0.8 0.4 0 3 2 1 0 0 2 0 2 4 6 8 mg Chl-a eq. m-3 4 6 8 Figure 10 36 Specific ingestion (µgC µg-1 ind C day-1) 3 nauplii cop < 200 µm cop > 200 µm 2 1 0 -1 0.01 0.1 1 Carbon weight (µg C ind-1 ) 10 100 Figure 11 37 Table 1. Abundance (individual m-3) of the different taxonomic groups in the fraction >200 µm. COPE- Copepods, NAU- Nauplii, APPAppendicularians, CHA- Chaetognata, DECL- Decapod larvae, EUL- Euphausiids larvae, POL-Polychaeta, CIRRL- Cirripedes larvae, JELLJellyfish, FISHE- Fish eggs, FISHL- Fish larvae, CLA- Cladocerans, OST- Ostracods, SIPHO- Siphonophors, DOL- Doliolids, AMPAmphipods, PTER- Pteropods, ECHIL- Echinoderm larvae. Station E1 E1 E1 E1 E1 E1 E1 E1 E1 E1 E1 E1 Month JAN FEB MAR APR MAY JUN JUL AUG SEP OCT NOV DEC COPE 268 1469 2456 782 2557 5596 8925 4625 12959 5317 3996 605 NAU 12 30 56 35 31 664 489 42 7 147 39 15 APP 218 305 1195 27 763 1027 265 265 10 63 14 5 CHA 3 3 3 63 38 77 21 56 3 - DECL 14 9 10 65 42 45 42 24 7 3 1 EUL 2 7 14 1 POL 1 45 45 7 12 14 10 14 17 7 3 2 CIRRL 922 1058 5 14 416 - JELL 1 49 13 12 21 87 21 3 3 - FISHE 15 8 10 6 10 3 56 6 8 FISHL 1 7 5 14 3 1 CLA 1 77 155 252 615 59 42 49 49 - OST 1 45 6 - SIPHO 15 360 20 454 161 108 91 164 112 8 2 DOL 7 203 817 171 685 11 - AMP 1 PTER 17 7 21 112 - ECHIL 5 14 31 3 - E2 E2 E2 E2 E2 E2 E2 E2 E2 E2 E2 E2 JAN FEB MAR APR MAY JUN JUL AUG SEP OCT NOV DEC 1074 5117 1146 926 2826 3647 1923 2878 4087 4136 2227 460 6 608 48 80 45 734 63 84 73 618 126 13 179 28 35 423 299 587 59 440 265 150 70 17 2 28 12 21 21 7 6 1 16 28 14 21 28 21 10 21 21 3 5 14 17 10 6 - 66 1 7 7 7 3 7 17 3 8 - 16760 80 49 - 1 3 7 5 70 26 63 7 52 8 1 1 24 3 7 2 3 - 7 3 - 56 134 231 9 7 119 38 - 7 - 15 133 141 119 19 35 56 147 - 14 91 999 629 475 53 - 1 - 3 14 - 10 5 63 2 14 3 - E3 E3 E3 E3 E3 E3 E3 E3 E3 E3 E3 JAN FEB MAR APR MAY JUN JUL AUG SEP OCT NOV 851 8251 3828 1058 3147 3376 2613 2417 3011 4464 2719 20 1816 286 119 35 84 234 56 63 482 117 1 70 576 318 3 171 161 398 517 447 162 4 7 9 10 21 21 28 3 45 21 11 17 10 9 3 3 1 7 14 10 3 21 7 21 6 161 10 10 7 10 14 - 1125 7 2 2 - 1 7 7 7 5 9 49 91 126 8 14 - 7 1 3 7 7 - 7 77 14 7 115 28 196 189 - 6 - 1 38 196 147 35 14 42 63 119 14 3 5 265 1188 314 2187 56 7 - 14 14 14 - 1 14 17 14 - 38 Table 2. Abundance (individual m-3) of the different taxonomic groups in the fraction <200 µm. NAU- Nauplii, COPE- Copepods, APP- Appendicularians, CIRRLCirripedes larvae. Station E1 E1 E1 E1 E1 E1 E1 E1 E1 E1 E1 E1 Month JAN FEB MAR APR MAY JUN JUL AUG SEP OCT NOV DEC NAU 2173 2480 2241 1062 4317 16306 12181 26715 18835 30271 12631 4692 COPE 391 548 1509 258 887 1146 1271 5882 145003 6239 3745 685 APP 295 108 267 42 112 279 28 140 35 70 49 8 CIRRL 189 14 - E2 E2 E2 E2 E2 E2 E2 E2 E2 E2 JAN FEB MAR APR MAY JUN JUL AUG SEP NOV 5669 5498 7769 1740 6658 8460 10263 17445 45780 15244 1235 1184 1921 545 814 2194 1446 4555 9334 4653 129 101 182 7 91 363 154 - 148 7 14 3 - E3 E3 E3 E3 E3 E3 E3 E3 E3 E3 E3 E3 JAN FEB MAR APR MAY JUN JUL AUG SEP OCT NOV DEC 4200 7140 14594 2522 12886 13106 9746 10249 21084 48666 17535 3138 813 1097 4443 660 1740 2746 831 5379 3577 8188 3486 1095 4 35 803 227 3 84 35 91 252 182 126 70 - 39 Table 3. Mean-square error (MSE) and parameters for each of the three types of functional responses for nauplii and cop <200 µm. Imax expressed as µg C µg-1 nauplii C d-1 and Cd and Kc as mg chl-a m-3. Model Type 1 Type 2 Type 3 Nauplii Cop <200 µm a Cd 0.49 3.17 0.645 1.83 Imax 1.56 1.179 MSE 0.102 0.265 a 0.65 0.94 Imax 1.72 1.58 MSE 0.102 0.262 a 0.96 0.78 kc 1.72 1.29 Imax 1.60 1.63 MSE 0.097 0.243 40