Completed Early Career Research Fellowship: Nicola Bye
advertisement

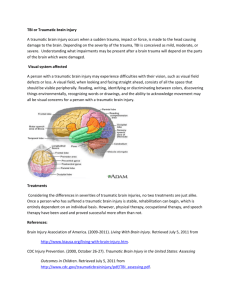
Completed Early Career Research Fellowship: Nicole Bye Host Organisation: National Trauma Research Institute (NTRI) TAC Funding: $326,270 Mentor: Associate Professor Cristina Morganti-Kossmann Fellowship Project: Enhancing endogenous neurogenesis with combined erthropoietin and growth factor treatment to promote tissue repair and neurological recovery following traumatic brain injury Background: Traumatic brain injury (TBI) is a devastating condition leading to progressive neuronal loss and consequent neurological deficit. Numerous clinical trials targeting neurotoxic cascades in TBI patients have failed pharmacological translation. Recently, a novel opportunity for therapeutic intervention has been identified with the discovery of resident neuronal stem cells in the adult brain that have the capacity to undergo neurogenesis to generate new neurons. Our preliminary studies have shown that a robust proliferation of neuronal stem cells occurs after experimental TBI, and that immature neurons migrate into damaged regions of the brain. However, these new neurons ultimately die off. Interestingly, other recent investigations have shown that neurogenesis can be supported in the naive brain or stroke-injured brain by treatment with specific growth factors, including erythropoietin (EPO) and brainderived neurotrophic factor (BDNF). Based on this work, our the proposed studies will explore whether treatment with EPO and BDNF, individually or combined, enhances the neurogenic response occurring after TBI, and also whether enhanced neurogenesis is correlated with improved neurological recovery in TBI mice. Overall, we hypothesise that administration of EPO and BDNF will improve motor and cognitive recovery following TBI by enhancing neurogenesis. Aim: The aim of this project is to determine whether treatment with EPO, BDNF, or EPO+BDNF, enhances neurogenesis and improves neurological recovery following TBI. Methods: These studies use a mouse model of focal brain injury that has been well established in our laboratory. This TBI model causes a contusion to the left cortex, with a long term neurological deficit that is quantified using specific sensorimotor tests. Mice subjected to TBI were treated with EPO, BDNF, EPO+BDNF or vehicle-control solution, administered for 2 weeks. To label stem cells proliferating in the brain, mice were injected with a compound (BrdU) that is incorporated into duplicating DNA in dividing cells. Cells containing BrdU can subsequently be visualised on brain tissue sections in conjunction with staining to identify immature and mature neurons, in order to assess their fate. Labelled cells are quantified and the numbers of new cells are compared between the different treatment groups in order to assess the effect of growth factor treatment on TBI-induced neurogenesis. Results: The results of this study to date demonstrate that treatment with EPO and/or BDNF improves neurological outcome following TBI, and that this effect is associated with an attenuated loss of neuronal tissue in the injured cortex. Experiments are ongoing test our hypothesis that treatment with EPO and/or BDNF will enhance neurogenesis after TBI, potentially contributing to improved functional recovery. Conclusion: The results of this study will indicate whether targeting neurogenesis is a strategy worth pursuing for the development of novel therapeutics. Furthermore, this project is directly relevant to human TBI, as it is investigating the use of a pharmacological paradigm that could potentially be implemented in TBI patients. Feedback on the Fellowship from Nicole Bye: “Receiving the TAC Early Career Researcher Fellowship has enabled me to continue pursuing my chosen research field of neuroregeneration after TBI. Without this funding, it would have been necessary for me to find employment elsewhere, which most likely would have meant shifting from neurotrauma research” “Importantly, over the past three years of the Fellowship I have strengthened my track record by increasing my senior author publications, successful grants and teaching experience. Furthermore, I am beginning to establish a reputation within the neurotrauma and neurogenesis research communities. Together, these improvements will support the continued growth of my career.” Publications: CRACK PJ, GOULD J, BYE N, ROSS S, ALI U, HABGOOD MD. MORGANTI-KOSSMANN C, SAUNDERS N, HERTZOG PJ. The genomic profile of the cerebral cortex after closed head injury in mice: effects of minocycline. Journal of Neural Transmission. 2009,116:1-12. SEMPLE BD, BYE N, ZIEBELL JM., MORGANTI-KOSSMANN MC. Deficiency of the chemokine receptor CXCR2 attenuates neutrophil infiltration and cortical damage following closed head injury. Neurobiol Disease. 2010;40:394-403. HELLEWELL SC, YAN EB, AGYAPOMAA DA, BYE N, MORGANTI-KOSSMANN MC. Posttraumatic hypoxia exacerbates brain tissue damage: Analysis of axonal injury and glial responses. J Neurotrauma. 2010;27(11):1997-2010. MORGANTI-KOSSMANN MC, SEMPLE B, ZIEBELL J, YAN E, BYE N, KOSSMANN T. Modulation of immune response by head injury. In: BERCZI I, ARNASON B, editors. Neuroimmune Biology: The Brain and host defence. Canada: Elsevier Science Manitoba; 2010 p.193-210. SEMPLE BD, BYE N, RANCAN M, ZIEBELL JM, MORGANTI-KOSSMANN MC. Role of CCL2 (MCP-1) in traumatic brain injury (TBI): evidence from severe TBI patients and CCL2-/- mice. Journal of Cerebral Blood Flow and Metabolism. 2010;30(4):769-82. MORGANTI-KOSSMANN MC, YAN E, BYE N. Animal models of traumatic brain injury: Is there an optimal model to reproduce human brain injury in the laboratory? Injury. 2010;41S:S10-S13. BYE N, CARRON S, HAN X, AGYAPOMAA D, NG SY, YAN E, ROSENFELD JV, MORGANTIKOSSMANN MC. Neurogenesis and glial proliferation are stimulated following diffuse traumatic brain injury in adult rats. J Neurosci Res. 2011;89(7):986-1000. BYE N, CARRON S, HAN X, AGYAPOMAA D, NG SY, YAN E, ROSENFELD JV, MORGANTIKOSSMANN MC. Neurogenesis and glial proliferation are stimulated following diffuse traumatic brain injury in adult rats. Journal of Neuroscience Research 2011. 89(7):986-1000. Presentations: RISTESKI M, BYE N, NG SY, SEMPLE B, MORGANTI-KOSSMANN MC. The role of monocyte chemoattractant protein-1 (MCP-1) in neurogenesis after traumatic brain injury (TBI). Trauma Melbourne; 2009 November 20-21, Melbourne, Australia. BYE N, TRAN M, NG S, MORGANTI-KOSSMANN MC. Characterising endogenous neurogenesis following experimental focal traumatic brain injury (TBI). 27th International Australasian Winter Conference on Brain Research; 2010 August 29 - September 2, Queenstown, New Zealand. SEMPLE B, BYE N, MALAKOOTI N, ZIEBELL J, MORGANTI-KOSSMANN MC. MCP-1 knockout mice exhibit improved neurological outcome, altered cytokine production, reduced macrophage infiltration and reduced lesion volume following focal traumatic brain injury. Joint National/International Neurotrauma Conference; 2009 September 7-11, Santa Barbara, USA. MORGANTI-KOSSMANN MC, SEMPLE B, BYE N, MALAKOOTI N, ZIEBELL J. Deficiency of the chemokine receptor CXCR2 attenuates neutrophil infiltration but has no effect on neurological recovery following closed head injury. Joint National/International Neurotrauma Conference; 2009 September 7-11, Santa Barbara, USA. ZIEBELL J, BYE N, SEMPLE B, KOSSMANN T, MORGANTI-KOSSMANN MC. Lack of functional Fas in TBI mice attenuates neurological deficit, cell death and lesion volume and is associated with altered cytokine patterns. Joint National/International Neurotrauma Conference; 2009 September 7-11, Santa Barbara, USA. BYE N, MURRAY L, TRAN M, NG S, MORGANTI-KOSSMANN MC. Characterising endogenous neurogenesis following experimental focal traumatic brain injury. Joint National/International Neurotrauma Conference; 2009 September 7-11, Santa Barbara, USA. SEMPLE B, BYE N, MALAKOOTI N, ZIEBELL J, MORGANTI-KOSSMANN MC. Deficiency of the chemokine receptor CXCR2 attenuates neutrophil infiltration but has no effect on neurological recovery following closed head injury. Trauma Melbourne; 2009 November 20-21, Melbourne, Australia. NG SY, BYE N, TRAN M, SEMPLE BD, KOSSMANN T, MORGANTI-KOSSMANN MC. Treatment with minocycline, an anti-inflammatory drug, improves neurological outcome but does not affect neurogenesis following focal traumatic brain injury in mice. Trauma Melbourne; 2009 November 20-21, Melbourne, Australia. BYE N. Characterising endogenous neurogenesis following experimental focal traumatic brain injury (TBI), and investigating the effect of treatment with minocycline. Trauma Melbourne; 2009 November 20-21, Melbourne, Australia. SEMPLE B, BYE N, MALAKOOTI N, ZIEBELL J, MORGANTI-KOSSMANN MC. MCP-1 knockout mice exhibit improved neurological outcome, altered cytokine production, reduced macrophage infiltration and reduced lesion volume following focal traumatic brain injury. Trauma Melbourne; 2009 November 20-21, Melbourne, Australia. SEMPLE BD, BYE N, ZIEBELL J, MORGANTI-KOSSMANN MC. Deficiency of the chemokine receptor CXCR2 attenuates neutrophil infiltration and lesion volume following closed head injury. Australian Neuroscience Society 30th Annual Meeting; 2010 January, Sydney, Australia. BYE N, NG SY, TRAN M, SEMPLE BD, KOSSMANN T, MORGANTI-KOSSMANN MC. Minocycline does not affect neurogenesis, but improves neurological outcome following traumatic brain injury in mice. Australian Neuroscience Society 30th Annual Meeting; 2010 January, Sydney, Australia. BYE N, SEMPLE BD, ROSENFELD JV, MORGANTI-KOSSMANN MC. Traumatic brain injury stimulates production of new immature neurons, but their survival may require exogenous neurotrophic support. Australian Neuroscience Society 31st Annual Meeting; 2011 January 31- February 3, Auckland, New Zealand. BYE N, ROSENFELD JV, MORGANTI-KOSSMANN MC. Traumatic brain injury stimulates production of new immature neurons, but their survival may require exogenous neurotrophic support. Trauma 2011, November 2011, Melbourne Australia. Bye N, Semple BD, Rosenfeld JV, Morganti-Kossmann MC. Traumatic brain injury stimulates production of new immature neurons, but their survival may require exogenous neurotrophic support. Australian Neuroscience Society 31st Annual Meeting, 2011 January, Auckland, New Zealand. HELLEWELL S, YAN E, BYE N, MORGANTI-KOSSMANN MC. Erythropoietin ameliorates axonal damage, attenuated macrophage infiltration, and restores motor function in a combined model of traumatic axonal injury and hypoxia. 10th International Neurotrauma Symposium (INTS), 2011 April 27-30, Shanghai, China. GOH CP, BYE N, MORGANTI-KOSSMANN MC, PUTZ U, TAN SS. Ndfip1 protects neurons from death following brain injury. Australian Neuroscience Society 31st Annual Meeting, 2011 January, Auckland, New Zealand. MORGANTI-KOSSMANN MC, SEMPLE BD, YAN E, FRUGIER T, HELLEWELL S, BYE N. Modulation of the immune response by traumatic brain injury. 10th International Neurotrauma Symposium (INTS), April 27-30, 2011, Shanghai,China. YAN E, SATGUNASEELAN L, HELLEWELL S, BYE N, ROSENFELD JV, COOPER DJ, MORGANTI-KOSSMANN MC. A hypoxic insult in patients with traumatic brain injury enhances cerebral inflammatory cytokines, serum biomarkers, and blood brain barrier dysfunction leading to unfavourable outcome. Trauma 2011, November 2011, Melbourne Australia. BYE N, NG SY, MORGANTI-KOSSMANN MC. Attenuation of microglial activation with minocycline is not associated with changes in neurogenesis after focal traumatic brain injury in adult mice. 2nd Australian Neurotrauma Symposium, October 2011, Hobart, Australia. Bye N, Ng SY, Semple BD, Morganti-Kossmann MC. Minocycline does not affect neurogenesis, but improves neurological outcome following focal traumatic brain injury in mice. 10th International Neurotrauma Symposium (INTS), 2011 April 27-30, Shanghai,China. BYE N, OEI D, SEMPLE BD, NG SY, MORGANTI-KOSSMANN MC. deficiency of the chemokine MCP-1 impedes the recruitment of subventricular zone neuroblasts into the pericontusional cortex after traumatic brain injury in adult mice. Australian Neuroscience Society 32nd Annual Meeting, January 2012, Gold Coast, Australia. MORGANTI-KOSSMANN MC, SATGUNASEELAN S, BYE N, ROSENFELD JV, YAN E. A hypoxic insult in patients with traumatic brain injury enhances cerebral inflammatory cytokines, serum biomarkers and blood-brain barrier dysfunction associated with unfavourable outcome. Australian Neuroscience Society 32nd Annual Meeting, January 2012, Gold Coast, Australia.