Unit 8 PRACTICE Test - with Answers
advertisement
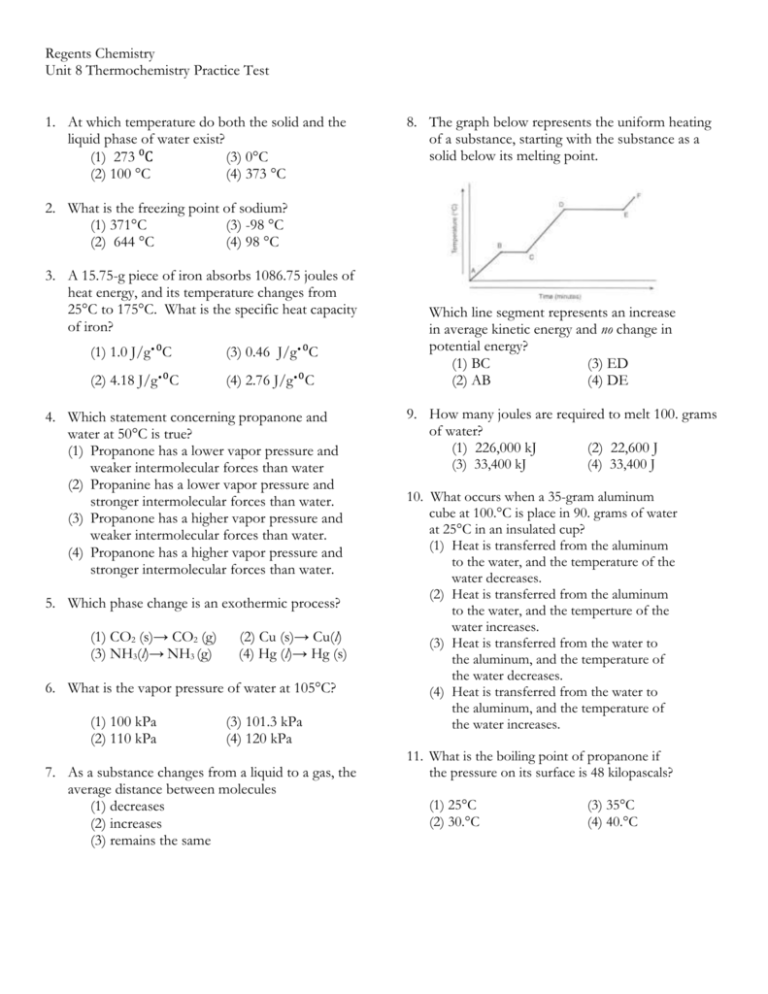
Regents Chemistry Unit 8 Thermochemistry Practice Test 1. At which temperature do both the solid and the liquid phase of water exist? (1) 273 ⁰C (3) 0°C (2) 100 °C (4) 373 °C 8. The graph below represents the uniform heating of a substance, starting with the substance as a solid below its melting point. 2. What is the freezing point of sodium? (1) 371°C (3) -98 °C (2) 644 °C (4) 98 °C 3. A 15.75-g piece of iron absorbs 1086.75 joules of heat energy, and its temperature changes from 25°C to 175°C. What is the specific heat capacity of iron? ∙ (1) 1.0 J/g ⁰C ∙ (2) 4.18 J/g ⁰C ∙ (4) 2.76 J/g∙⁰C (3) 0.46 J/g ⁰C 4. Which statement concerning propanone and water at 50°C is true? (1) Propanone has a lower vapor pressure and weaker intermolecular forces than water (2) Propanine has a lower vapor pressure and stronger intermolecular forces than water. (3) Propanone has a higher vapor pressure and weaker intermolecular forces than water. (4) Propanone has a higher vapor pressure and stronger intermolecular forces than water. 5. Which phase change is an exothermic process? (1) CO2 (s)→ CO2 (g) (3) NH3(l)→ NH3 (g) (2) Cu (s)→ Cu(l) (4) Hg (l)→ Hg (s) 6. What is the vapor pressure of water at 105°C? (1) 100 kPa (2) 110 kPa (3) 101.3 kPa (4) 120 kPa 7. As a substance changes from a liquid to a gas, the average distance between molecules (1) decreases (2) increases (3) remains the same (3) remains the sam Which line segment represents an increase in average kinetic energy and no change in potential energy? (1) BC (3) ED (2) AB (4) DE 9. How many joules are required to melt 100. grams of water? (1) 226,000 kJ (2) 22,600 J (3) 33,400 kJ (4) 33,400 J 10. What occurs when a 35-gram aluminum cube at 100.°C is place in 90. grams of water at 25°C in an insulated cup? (1) Heat is transferred from the aluminum to the water, and the temperature of the water decreases. (2) Heat is transferred from the aluminum to the water, and the temperture of the water increases. (3) Heat is transferred from the water to the aluminum, and the temperature of the water decreases. (4) Heat is transferred from the water to the aluminum, and the temperature of the water increases. 11. What is the boiling point of propanone if the pressure on its surface is 48 kilopascals? (1) 25°C (2) 30.°C (3) 35°C (4) 40.°C 12. The boiling point of a liquid is the temperature at which the vapor pressure of the liquid is equal to the pressure on the surface of the liquid. The heat of vaporization of ethanol is 838 joules per gram. A sample of ethanol has a mass of 65.0 grams and is boiling at 1.00 atmosphere. (a) Based on Table H, what is the temperature of this sample of ethanol? ______ (b) Calculate the minimum amount of heat required to completely vaporize this sample of ethanol. Your response must include both a correct numerical setup and the calculated result. 13. A 500.-gram sample of liquid water is set on a stove to boil. (a) Assuming that all the energy from the stove is uniformly added, how many joules must be absorbed by the water to completely change all of the liquid water to gaseous water at the boiling point? Your response must include both a correct numerical setup and the calculated result. (b) Calculate the moles of water in the sample. Show the numerical set-up and record the answer according to the rules of significant figures. 14. Carbon dioxide is a nonpolar substance that sublimes at room temperature. (a) Explain, in terms of intermolecular forces, why carbon dioxide sublimes. (b) Write the equation that shows solid carbon dioxide subliming. Include states and the energy term. (c) Describe what happens to the potential energy of the carbon dioxide molecules during this phase change. 15. Heat is added to a 200.-gram sample of H2O(s) to melt the sample at 0°C. Then the resulting H2O(ℓ) is heated to a final temperature of 65°C. (a) Determine the total amount of heat required to completely melt the sample. (b) Show a numerical setup for calculating the total amount of heat required to raise the temperature of the H2O(ℓ) from 0°C to its final temperature. (c) Compare the amount of heat required to vaporize a 200.-gram sample of H2O(ℓ) at its boiling point to the amount of heat required to melt a 200.-gram sample of H2O(s) at its melting point. Use the following information to answer questions 16 through 18. The temperature of a sample of a substance is increased from 20.°C to 160.°C as the sample absorbs heat at a constant rate of 15 kilojoules per minute at standard pressure. The graph below represents the relationship between temperature and time as the sample is heated. 16. What is the boiling point of the sample? 17. What is the total time this sample is in the liquid phase, only? 18. Determine the total amount of heat required, in kJ, to completely melt this sample at its melting point. ANSWERS 1. 3 2. 4 3. 3 4. 3 5. 4 6. 4 7. 2 8. 2 9. 4 10. 2 11. 3(3) remains the sam 12. (a) 80°C (b) q = mHv = (65.0)(838) = 54,470 J 13. (a) q = mHv = (500.)(2260) = 1,130,000 J (b) moles = given mass/GFM = 500/18 = 28 mol 14. (a) Carbon dioxide has very weak intermolecular forces. (b) CO2(s) + energy CO2(g) (c) The potential energy of the CO2 is increasing. 15. (a) q = mHf = (200)(334) = 66,800 J (b) q = mC∆T = (200)(4.18)(65) = 54,340 J (c) The heat required to vaporize a sample of water is greater than the heat required to melt it. 16. 120°C 17. 3 minutes 18. (2 min)(15 kJ/min) = 30 kJ