Sample Retrosynthesis of an Alkene
advertisement

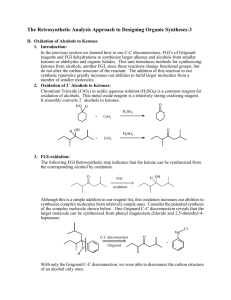
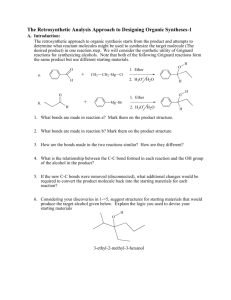
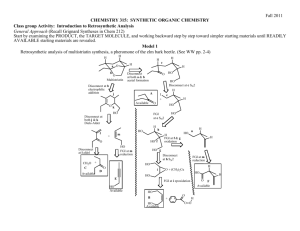
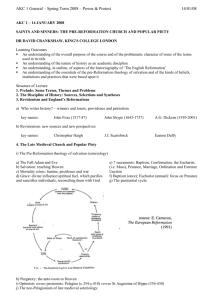
D1 V D. The Retrosynthetic Analysis Approach to Designing Organic Syntheses A. Introduction: The retrosynthetic approach to organic synthesis starts from the product and attempts to determine what reactant molecules might be used to synthesize the target molecule (The desired product) in one reaction step. We will consider the synthetic utility of Grignard reactions for synthesizing alcohols. Note that both of the following Grignard reactions form the same product but use different starting materials. O 1. Ether + a. CH3 CH2 Mg Cl H 2. H3O+ H2O O H H H O 1. Ether + b. O Mg Br 2. H3O+ H2O H H 1. What bonds are made in reaction a? Mark them on the product structure. 2. What bonds are made in reaction b? Mark them on the product structure. 3. How are the bonds made in the two reactions similar? How are they different? 4. What is the relationship between the C-C bond formed in each reaction and the OH group of the alcohol in the product? 5. If the new C-C bonds were removed (disconnected), what additional changes would be required to convert the product molecule back into the starting materials for each reaction? 6. Considering your discoveries in 1->5, suggest structures for starting materials that would produce the target alcohol given below. Explain the logic you used to devise your starting materials H O 3-ethyl-2-methyl-3-hexanol V D: Retro Synthetic Analysis D2 B. Synthesis of Alcohols: C-C Disconnections 1. Retrosynthetic Analysis Look at the product molecule, find the alcohol OH and identify the bonds around the carbon holding the OH. These are the bonds that could have been made by a Grignard reaction. Disconnect each bond (C-C disconnections) in turn to reveal potential precursors for the product. Then identify the reaction that will reveal the precursors of the Grignard reagent. In the terminology of Retrosynthetic Analysis, a process such as the formation of the Grignard reagent is a Functional Group Interconversion (FGI) since it changes the nature of the functional group but does not change the carbon structure. So the retrosynthetic step for formation of an organometallic reagent is symbolized by FGI Grig, since it is the formation of a Grignard reagent. Full Retrosynthetic Analysis for the synthesis of 3-ethyl-2-methyl-3-hexanol (Starting materials in bold) H O b. O a. a. c. synthon synthon C-C Disconnection O Mg Cl FGI Grig Cl + MgÞ c. C-C Disconnections b. C-C Disconnections O O synthon O synthon CH3 Mg Br synthon FGI Grig Br + MgÞ CH2 synthon O FGI Grig CH3 CH2 Mg Cl CH3 CH2 Cl + MgÞ 2. Synthesis based on retrosynthetic analysis b: O H O Br + MgÞ Dry Ether 1. Dry Ether Mg Br 2. H2SO4/H2O D3 V D: Retro Synthetic Analysis 3. An Additional C-C Disconnection Example b. a. O a. c. CH3 Mg Cl FGI Grig CH3 Cl + MgÞ C-C Disconnection O H b. C-C Disconnections c. C-C Disconnections O FGI Grig CH3 CH2 Mg Cl CH3 CH2 Cl + MgÞ O FGI Grig Mg + MgÞ Cl Cl 4. Synthesis based on retrosynthetic analysis c: O 1. Dry Ether Dry + Cl MgÞ Ether Mg 2. H2SO4/H2O Cl O H 5. Class Discussion Problem on Alcohol Synthesis: Use Retrosynthetic Analysis to devise a synthetic pathway for the following compound from smaller molecules. O H Then write a complete synthetic path, including reagents and reaction conditions based on your retrosynthetic analysis. V D: Retro Synthetic Analysis D4 6. Out of Class Exercises on C-C disconnections in alcohol syntheses. For our next lab discussion period, use retrosynthetic analysis to devise at least two syntheses for the following compounds. H O CH3 N CH3 O H Then write complete synthetic paths, including reagents and reaction conditions based on your retrosynthetic analyses. Be prepared to discuss both the retroanalysis and the synthesis. D5 V D: Retro Synthetic Analysis C. Alkene Synthesis by Dehydration of Alcohols 1. Dehydration of Alcohols: Dehydration of alcohols is induced by acidic conditions and (as we will see later) usually occurs by a 2-step mechanism. O H H2SO4 or H3PO4 a + b Major Product a. What bonds are made and broken in forming product a? Mark them on the product structure. b. What bonds are made and broken in forming product b? Mark them on the product structure. c. How do the bonds made and broken in the two reactions similar? How are they different? d. What is the relationship between each bond made or broken and the OH group of the alcohol reactant? e. If the new bonds were removed (disconnected), what additional changes would be required to convert each product molecule back into the starting material? f. Considering your discoveries in 1->5, suggest structures for starting materials that would produce the target alkene given below. Explain the logic you used to devise your starting materials V D: Retro Synthetic Analysis D6 2. Retro Synthetic Step: FGI-Dehydration Dehydration is another reaction that changes the functional group without altering the sequence of the carbon chain. The retrosynthetic step is designated as FGI dehydration. Usually FGI dehydrations can reveal two possible precursor compounds. H O FGI Dehydration OR FGI O H Dehydration Potential precursor alcohols can be revealed by adding an OH to either end of the C=C. The choice of end will depend upon potential competing products. Sample Retrosynthesis of an Alkene Cl-Mg b. O a. FGI a. C-C H Dehydration H O H C-C Cl Mgo Grignard FGI-M Cl Grignard FGI-M b. FGI Dehydration MgCl O O 2 other C-C's possible Mgo 3. Out of Class Application: For our next lab discussion period devise at least 2 different syntheses for each of the following compounds from simple monosubstituted aromatic compounds and nonaromatic compounds with four or fewer carbon atoms. Then write complete synthetic paths, including reagents and reaction conditions based on your retrosynthetic analyses. Be prepared to discuss both the retroanalysis and the synthesis. D7 V D: Retro Synthetic Analysis D. Oxidation of Alcohols to Ketones 1. Introduction: In the previous section we learned how to use C-C disconnections, FGI’s of Grignard reagents and FGI dehydrations to synthesize larger alkenes and alcohols from smaller ketones or aldehydes and organic halides. This unit introduces methods for synthesizing ketones from alcohols, another FGI, since these reactions change functional groups, but do not alter the carbon structure of the reactant. The addition of this reaction to our synthetic repertoire greatly increases our abilities to build larger molecules from a number of smaller molecules. 2. Oxidation of 2˚ Alcohols to Ketones: Chromium Trioxide (CrO3) in acidic aqueous solution (H2SO4) is a common reagent for oxidation of alcohols. This metal oxide reagent is a relatively strong oxidizing reagent. It smoothly converts 2˚ alcohols to ketones. O HO H H2 SO4 + CrO3 H OH O H2 SO4 + CrO3 3. FGI-oxidation: The following FGI Retrosynthetic step indicates that the ketone can be synthesized from the corresponding alcohol by oxidation. O H OH FGI oxidation Although this is a simple addition to our reagent list, this oxidation increases our abilities to synthesize complex molecules from relatively simple ones. Consider the potential synthesis of the complex molecule shown below. One Grignard C-C disconnection reveals that the target molecule can be synthesized from phenyl magnesium chloride and 2,5-dimethyl-4heptanone. Cl Mg C-C disconnection O Grignard O + H With only the Grignard C-C disconnection, we were able to disconnect the carbon structure of an alcohol only once. V D: Retro Synthetic Analysis D8 However, we now see that the ketone precursor revealed by the above disconnection can be synthesized from a 2˚ alcohol as indicated on page 80 and reproduced below: O H OH FGI oxidation This FGI reveals a compound that can be further disconnected with a Grignard C-C to reveal even simpler synthetic precursors. H OH O C-C disconnection Grignard Mg + Br H If we recall that Grignard Reagents can be synthesized from alkyl halides, we can look at the entire synthetic pathway as a set of disconnections and FGI's. The starting materials are enclosed in rectangles. Mg Cl O C-C disconnection O + Grignard H FGI-M FGI Oxidation O H OH H C-C disconnection + + Mgo Grignard Mg Cl Br FGI-M Br + Mgo D9 V D: Retro Synthetic Analysis 4. Out of Class Application: For our next lab discussion period use retrosynthetic analysis to devise syntheses for the following compounds from monosubstituted aromatic compounds and non-aromatic compounds with four or fewer carbon atoms. OH O Then write complete synthetic paths, including reagents and reaction conditions based on your retrosynthetic analyses. Be prepared to discuss both the retroanalysis and the synthesis.