Table S1: Summary of metabolite ions that showed significant
advertisement
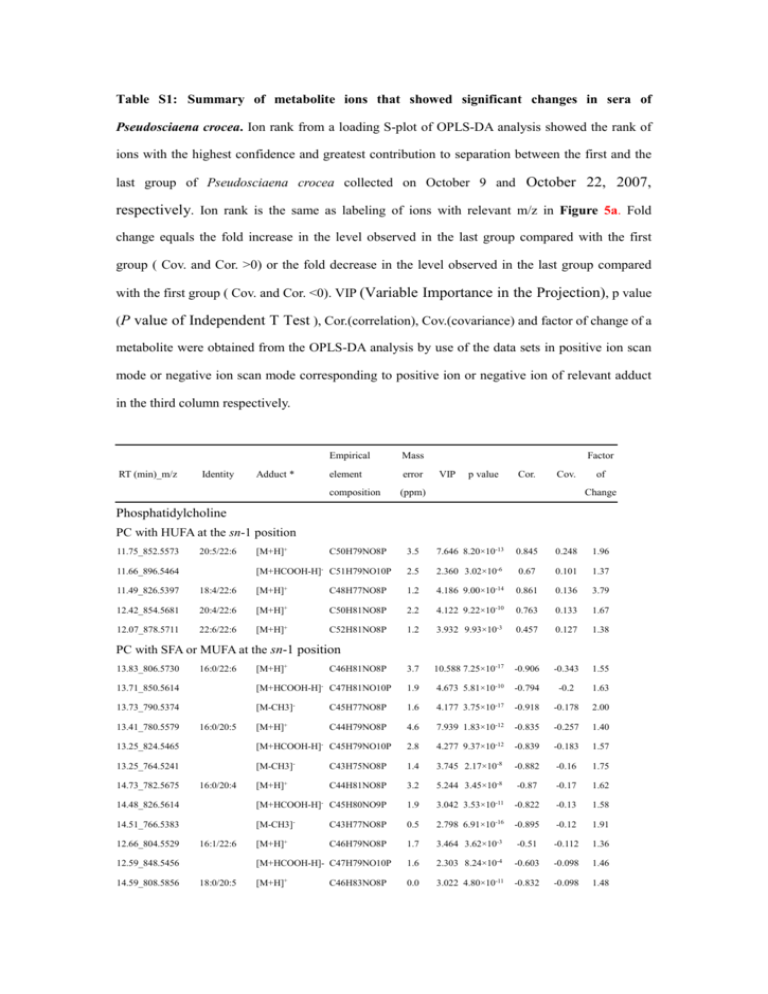
Table S1: Summary of metabolite ions that showed significant changes in sera of Pseudosciaena crocea. Ion rank from a loading S-plot of OPLS-DA analysis showed the rank of ions with the highest confidence and greatest contribution to separation between the first and the last group of Pseudosciaena crocea collected on October 9 and October 22, 2007, respectively. Ion rank is the same as labeling of ions with relevant m/z in Figure 5a. Fold change equals the fold increase in the level observed in the last group compared with the first group ( Cov. and Cor. >0) or the fold decrease in the level observed in the last group compared with the first group ( Cov. and Cor. <0). VIP (Variable Importance in the Projection), p value (P value of Independent T Test ), Cor.(correlation), Cov.(covariance) and factor of change of a metabolite were obtained from the OPLS-DA analysis by use of the data sets in positive ion scan mode or negative ion scan mode corresponding to positive ion or negative ion of relevant adduct in the third column respectively. Empirical RT (min)_m/z Identity Adduct * Mass element error composition (ppm) Factor VIP p value Cor. Cov. of Change Phosphatidylcholine PC with HUFA at the sn-1 position 11.75_852.5573 20:5/22:6 [M+H]+ 3.5 7.646 8.20×10-13 [M+HCOOH-H] C51H79NO10P 2.5 2.360 3.02×10 -6 C50H79NO8P - 11.66_896.5464 0.845 0.248 1.96 0.67 0.101 1.37 11.49_826.5397 18:4/22:6 [M+H]+ C48H77NO8P 1.2 4.186 9.00×10-14 0.861 0.136 3.79 12.42_854.5681 20:4/22:6 [M+H]+ C50H81NO8P 2.2 4.122 9.22×10-10 0.763 0.133 1.67 12.07_878.5711 22:6/22:6 [M+H]+ C52H81NO8P 1.2 3.932 9.93×10-3 0.457 0.127 1.38 3.7 10.588 7.25×10-17 -0.906 -0.343 1.55 1.9 -10 -0.794 -0.2 1.63 4.177 3.75×10-17 -0.918 -0.178 2.00 4.6 7.939 1.83×10-12 -0.835 -0.257 1.40 PC with SFA or MUFA at the sn-1 position 13.83_806.5730 16:0/22:6 [M+H]+ C46H81NO8P - 13.71_850.5614 [M+HCOOH-H] C47H81NO10P 13.73_790.5374 [M-CH3]- 13.41_780.5579 [M+H]+ 16:0/20:5 C45H77NO8P C44H79NO8P 1.6 4.673 5.81×10 13.25_824.5465 [M+HCOOH-H]- C45H79NO10P 2.8 4.277 9.37×10-12 -0.839 -0.183 1.57 13.25_764.5241 [M-CH3]- C43H75NO8P 1.4 3.745 2.17×10-8 -0.882 -0.16 1.75 [M+H]+ C44H81NO8P 3.2 5.244 3.45×10-8 -0.87 -0.17 1.62 14.48_826.5614 [M+HCOOH-H]- C45H80NO9P 1.9 3.042 3.53×10-11 -0.822 -0.13 1.58 14.51_766.5383 [M-CH3]- 0.5 2.798 6.91×10-16 -0.895 -0.12 1.91 12.66_804.5529 [M+H]+ 3.464 3.62×10-3 -0.51 -0.112 1.36 2.303 8.24×10-4 -0.603 -0.098 1.46 3.022 4.80×10-11 -0.832 -0.098 1.48 14.73_782.5675 16:0/20:4 16:1/22:6 12.59_848.5456 14.59_808.5856 C43H77NO8P C46H79NO8P [M+HCOOH-H]- C47H79NO10P 18:0/20:5 + [M+H] C46H83NO8P 1.7 1.6 0.0 15.28_808.5853 16:0/22:5 [M+H]+ C46H83NO8P 0.4 2.912 1.38×10-3 -0.584 -0.094 1.63 2.8 5.528 9.23×10-8 -0.818 -0.179 1.73 3.1 3.096 2.72×10-6 -0.78 -0.132 1.77 2.423 2.4×10-11 -0.799 -0.104 1.76 3.20×10-7 -0.803 -0.098 1.75 PC with plasmalogen at the sn-1 position 15.21_792.5885 p-16:0/22:5 [M+H]+ C46H83NO7P 14.93_836.5832 [M+HCOOH-H]- 14.91_776.5615 [M-CH3]- 14.63_766.5762 [M+H]+ C44H80NO7P 2.2 3.014 [M+H]+ C30H51NO7P 3.0 7.289 3.70×10-10 0.789 0.236 4.73 6.77_612.3323 [M+HCOOH-H]- C31H51NO9P 3.6 3.688 1.15×10-12 0.858 0.158 4.30 6.78_552.3079 [M-CH3]- C29H47NO7P 2.0 8.207 3.92×10-15 0.896 0.351 3.11 [M+H]+ C26H55NO7P 4.0 3.522 1.61×10-5 0.7 0.114 1.98 2.4 2.288 1.00×10-2 0.442 0.098 1.40 3.502 3.62×10-11 0.809 0.113 2.08 -13 0.857 0.192 1.93 1.60×10-3 0.489 0.109 1.61 p-16:0/20:4 C47H83NO9P C45H79NO7P 2.7 Lysophosphatidyl choline 6.96_568.3386 9.14_524.3695 22:6 18:0 8.99_508.3391 [M-CH3]- 8.08_522.3543 [M+H]+ 18:1 C25H51NO7P C26H53NO7P - 7.93_506.3228 [M-CH3] 7.79_496.3383 16:0 [M+H]+ C24H51NO7P 4.0 3.372 6.31_542.3234 20:5 [M+H]+ C28H49NO7P 2.8 2.899 2.40×10-14 0.818 0.094 4.96 [M-CH3]- C27H45NO7P 4.2 4.077 2.46×10-14 0.891 0.174 3.73 6.15_526.2912 C25H49NO7P 3.3 3.8 4.486 3.24×10 6.93_528.3070 20:4 [M-CH3]- C27H47NO7P 3.8 3.055 5.20×10-14 0.876 0.131 3.60 7.31_554.3249 22:5 [M-CH3]- C29H49NO7P 0.5 2.518 4.70×10-14 0.918 0.108 6.04 C21H35O5 4.6 3.62 2.84×10-9 -0.898 -0.155 1.90 C26H44NO7S 2.5 3.105 4.59×10-4 0.342 0.133 7.18 Corticosterone metabolite 9.71_367.2501 cortol [M -H]- Cholic acid metabolite 2.69_514.2826 taurocholic acid [M -H]-